Colorectal Cancer
Integrative
Care
Guidelines
Examples
Integrative
Therapies
Treatment
Terrain
Wellness
Risk
Integrative
Programs
Surgery
Commentary
More
Information
Quick ReferenceUpdated February 2021: Open a 2-page quick reference summary of the therapies best supported by evidence for use with colorectal cancer: |
Key Points
|
Authors
|
Colorectal cancer is a term used to include several types of cancers of the colon and/or rectum. Common types of colorectal cancers:1
- Adenocarcinomas of the colon and rectum
- Gastrointestinal carcinoid tumors
- Primary colorectal lymphomas
- Gastrointestinal stromal tumors
- Leiomyosarcomas
- Melanomas of the colon or rectum
The evidence presented here for screening, diagnosis, treatment and reducing risk relates to carcinomas, of which the great majority are adenocarcinomas. The other cancer types are much less common, and behave quite differently.
Colorectal cancer begins when healthy cells in the lining of the colon or rectum change and grow out of control. These cells form a mass called a tumor, which can be cancerous or benign. A cancerous tumor is malignant, meaning it can grow and spread to other parts of the body. A benign tumor can grow but will not spread. These changes usually take years to develop.2
Colorectal Cancer: Signs, Symptoms and ScreeningSigns and symptoms from the American Cancer Society:3
Because colorectal cancers can bleed into the intestinal tract, signs of anemia may also be an early indicator of colorectal cancer. Signs of anemia:
A rectal or abdominal mass is also a possible sign The US Preventive Services Task Force recommends screening for all adults aged 50 to 75. Colorectal cancer screening strategies include stool tests, flexible sigmoidoscopy, colonoscopy, and CT colonography (virtual colonoscopy). Those with an increased risk may need to be tested earlier than age 50 or more often than other people. Increased risk factors:
Also see the QCancer®(15yr,colorectal) risk calculator. |
Of cancers that affect both men and women, colorectal cancer is the second leading cancer killer in the United States. It is most often found in people who are 50 years old or older.4 However, incidence is increasing in younger adults and declining in older age groups.5
There are many possible reasons for the fewer early-stage diagnoses in adults under 50, such as these:
- Younger adults may be less likely to report symptoms promptly or to have medical insurance than older adults, which may lead to initial diagnosis at a later stage.6
- Younger adults may also present more often with symptoms outside the national referral guidelines, leading to fewer prompt referrals for colorectal cancer assessment.7
Early detection, allowing for early treatment, is very important with colorectal cancer. Treatment is often most effective in small localized cancer. When the cancer is diagnosed in advanced stages, it is often not operable, which often means a lower chance of survival.8 Suggestions for detecting cancer early:
- Follow all screening guidelines, such as from What Should I Know About Screening? from the Centers for Disease Control and Prevention (also see at right).
- If you have a family history or any symptoms of colorectal cancer, ask your physician about more aggressive screening.
See a list of colorectal cancer signs and symptoms in a sidebar.
Integrative Care in Colorectal Cancer
Before investigating integrative care in colorectal cancer, we recommend reviewing integrative cancer care in general.
Our goal is to help you live as well as you can for as long as you can. We provide information about using an optimal integrative combination of conventional and complementary therapies and approaches. In this handbook, we present a wide range of complementary therapies that have been studied for their effectiveness in colorectal cancer.
We give a brief description of what’s known about these therapies. We also group natural products and off-label and novel therapies (which we call ONCAs) according to safety, effectiveness and ease of access.
We consider the cancer within the context of the whole person. Cancers are composed of cells that divide without stopping. Some divide slowly, others quickly. Some are more invasive than others. But they don’t act independently of everything going on in your body.
Your body terrain—the internal environment that is influenced by external factors such as the foods you eat, the chemicals you contact, light and radiation you’re exposed to, plus internal factors such as stress hormones, sex hormones, your fitness, feelings of being loved and your sense of purpose—can set the stage for whether cancer will grow and thrive. Will the cancer find the chemical and biological terrain that promotes growth or not? You have more control over this than you may realize.
Your body terrain can influence the tumor microenvironment—the biochemical and physical interaction of cancerous and noncancerous cells. The microenvironment makes the cancer either more or less likely to grow and spread. (See Body Terrain and the Tumor Microenvironment.) You may be able to improve your body terrain with an integrative approach.
A 2018 article in The Journal of Alternative and Complementary Medicine provides an excellent overview of integrative therapies: Integrative treatment for colorectal cancer: a comprehensive approach.9
Healing and Curing
Many of the integrative approaches in this handbook promote healing, which is not the same as curing. Healing is an inner process through which a person becomes whole. Healing can take place at physical, emotional, mental and spiritual levels. An example of physical healing is when a surgical incision heals.
A cure is a successful medical treatment that removes all evidence of disease and allows the person who previously had cancer to live as long as he or she would have lived without cancer. For any cure to work, your healing power must be sufficient to enable recovery. Healing goes beyond curing and may happen whether or not the cancer is cured. Although the capacity to heal physically is necessary to any successful cure, healing can also take place on deeper levels, whether or not physical recovery occurs.
Whether or not your colorectal cancer is curable, healing is always possible and may provide these benefits:
- Slow the cancer’s growth and spread
- Improve survival
- Reduce the risk of recurrence
- Alleviate symptoms and side effects
- Improve your overall well-being
Healing will help you feel whole regardless of how cancer may change your body or your life.
Use the information you find here to guide your choices in healing. Share this information with your cancer care team. We provide the evidence to date behind the therapies, and we group natural products and ONCAs—off-label, overlooked and novel cancer approaches—by their safety and strength of evidence to make it easier for your team to discern the best options for you and your specific situation.
Learn More
Integrative Approaches and SurgerySurgery may be part of the recommended treatment for this cancer type. We provide helpful information about how integrative approaches can coordinate with surgery below in the section titled Surgery and Colorectal Cancer. |
We recommend these resources to introduce you to conventional therapies and the science behind them:
- National Cancer Institute:
- Cancer.net: Colorectal Cancer
Knowing how your cancer behaves will influence the type of testing and treatment used, prepare you for possible treatment side effects and guide you in steps to prevent or minimize these effects. It will help you understand and choose the complementary therapies and lifestyle approaches that may enhance your conventional treatment, manage side effects and improve your quality of your life.
You can also prepare your home team for what to expect. You can plan ahead to line up the support you may need. You can anticipate side effects and work to minimize them even before treatment starts. Finally, learning what to expect allows you to prepare mentally and spiritually to catalyze your resilience for facing the weeks and months to come.
You may read “the five-year survival for this cancer is X percent.” That means that this percentage of people survive at least five years. But expected survival doesn’t show the range of survival—which can vary from months to decades. We know many people who have lived far beyond the expectation. Getting healthier with cancer—and skillful use of conventional and complementary therapies—may help extend your life. It will very likely improve the quality of your life. There is nothing wrong with hope.
Clinical Practice Guidelines
- National Comprehensive Cancer Network:
- Professional Guidelines (Login required):
- Guidelines for Patients:
- American Society of Clinical Oncology: Gastrointestinal Cancer
Screening Guidelines
For Health Professionals: Surveillance ScheduleRecommended schedule of surveillance for colon and rectal cancer (AJCC stage I (at increased risk for recurrencea), stage II, stage III, and stage IV (when isolated metastases are resected for cure)10 Colon
Rectumb
Notes and DefinitionsAJCC = American Joint Committee on Cancer
|
- Clinical Guidelines Committee of the American College of Physicians: Screening for Colorectal Cancer in Asymptomatic Average-Risk Adults: A Guidance Statement From the American College of Physicians (2019)
- British Medical Journal: Colorectal cancer screening with faecal immunochemical testing, sigmoidoscopy or colonoscopy: a clinical practice guideline (2019)
- The American College of Gastroenterology: ACG Clinical Guidelines: Colorectal Cancer Screening 2021
Guidelines following Curative Treatment
- Clinical Practice Guidelines Committee of the American Society of Colon and Rectal Surgeons: Practice guideline for the surveillance of patients after curative treatment of colon and rectal cancer
Other Professional Recommendations
The US Preventive Services Task Force recommends initiating low-dose (81 mg) aspirin use for the primary prevention of cardiovascular disease (CVD) and colorectal cancer in adults aged 50 to 59 years who have a 10 percent or greater 10-year CVD risk, are not at increased risk for bleeding, have a life expectancy of at least 10 years, and are willing to take low-dose aspirin daily for at least 10 years.11 Use is not recommended for others, as risks from taking aspirin may outweigh benefits. Even those not at risk may experience catastrophic gastrointestinal bleeding.
Examples of Integrative Approaches
Bastyr Integrative Oncology Research Center (BIORC)
Between 2009 and 2014, 704 cancer patients were enrolled in an observational study at Bastyr Integrative Oncology Research Center (BIORC). Cancer types included lung, breast, ovarian, colon, pancreatic, brain and skin cancers. One-third of those patients had advanced cancer. BIORC used intravenous (IV) high-dose vitamin C, IV artesunate, oral curcumin, green tea and turkey tail mushrooms (Trametes versicolor).12
Preliminary results reported in 2013 from the BIORC are promising, as reported by BIORC's medical director and BCCT advisor Leanna J. Standish, PhD, ND, LAc, FABNO: “For eight patients with stage 4 colon cancer, BIORC reported an 80 percent survival rate after three years, compared with 15 percent from a group at Seattle Cancer Care Alliance.”13
"Our patients are doing better than national averages," says Dr. Standish, a professor at Bastyr University and the University of Washington. "We don't know why. Maybe they would have done better, or maybe there's something about our treatment."
Similarly, of 12 BIORC patients with stage 4 lung cancer, 64 percent were alive after three years, compared with 15 percent from Seattle Cancer Care and three percent from a national data group. Limitations in most data sets make exact comparisons difficult.
Life Over Cancer System
The Block Center for Integrative Cancer Treatment (BCICT), founded by integrative oncologist and BCCT advisor Keith Block, MD, offers a comprehensive cancer treatment program combining conventional treatments—often delivered in novel ways, such as according to circadian rhythms—along with nutrition and supplementation, fitness and mind-spirit instruction. The program is highly individualized and provides care to people with any kind of cancer.
Dr. Keith Block reported a case study of a 49-year-old man with colorectal cancer diagnosed in December 2002. Three years post diagnosis, after two surgeries and 12 chemotherapy cycles, he was in remission. In January 2006, he was diagnosed with stage 4 metastases.
Dr. Block prescribed an individualized program to enhance treatment tolerability, reduce treatment toxicity and boost treatment effectiveness through molecular profile testing. The Life Over Cancer program includes these therapies:
- Therapeutic nutrition to boost stamina, counter fatigue and reduce chemotherapy side effects
- Mind-spirit interventions to reduce stress
- Exercise to build strength and fitness
- Chronomodulated chemotherapy via a portable pump which deliversboth chemotherapy drugs and intravenous supplemental nutrients
The patient's outcome:
- He was able to stay active because of the portable pump.
- His scans improved.
- He reported no troubling side effects, and he tolerated the chemotherapy so well he did not need to reduce the dose.
- After seven chronotherapy sessions (five fewer than would have been used conventionally), he showed no evidence of disease (NED).
- As of the writing in 2009, seven years after his original diagnosis and three years after diagnosis of stage 4 metastases, the patient was in complete remission and back at work.
- For comparison, in the USA, colon cancer has a five-year life relative survival rate of 63 percent across all stages, and a 14 percent rate for distant spread (metastases).14
This approach is discussed in detail in a 2018 article, including Block’s use of three spheres of intervention: improving lifestyle, regulating biology, and enhancing treatment.15
Integrative Programs, Protocols and Medical Systems
| For more information about programs and protocols, see our Integrative Programs and Protocols page. |
Traditional Medicine TherapiesThroughout this summary, you will find examples of therapies used by, and in many cases created by, traditional medical systems. Foods and herbs such as medicinal mushrooms, soy and curcumin are part of traditional systems. Evidence shows that herbs used in traditional Chinese medicine (TCM) may help in maintaining immune function in women with ovarian cancer, for comparison. Mind-body practices such as mindfulness meditation and yoga also have roots in these systems. Acupuncture and electroacupuncture, another approach that is part of the Chinese and Korean medicine traditions, is used to relieve many symptoms during and following treatment. Electroacupunture even improved recovery of gastrointestinal function following surgery for colorectal cancer. See details below in Managing Side Effects and Promoting Wellness. |
- Programs and protocols
- Alschuler & Gazella complementary approaches16
- Block program17
- Cohen & Jefferies Mix of Six anticancer practices18
- Lemole, Mehta & McKee colorectal cancer protocol19
- McKinney colorectal cancer protocol20
- Parmar & Kazcor treatment plans21
- Traditional systems
- Ayurveda
- Traditional Chinese medicine22
- Traditional Korean medicine23
Integrative Therapies in Colorectal Cancer
7 Healing Practices: The Foundation
Top 5 Lifestyle Interventions following Colorectal Cancer TreatmentThe authors of After Cancer Care: The Definitive Self-Care Guide to Getting and Staying Well for Patients with Cancer recommend these lifestyle interventions,24 which we’ve matched to the 7 Healing Practices:
|
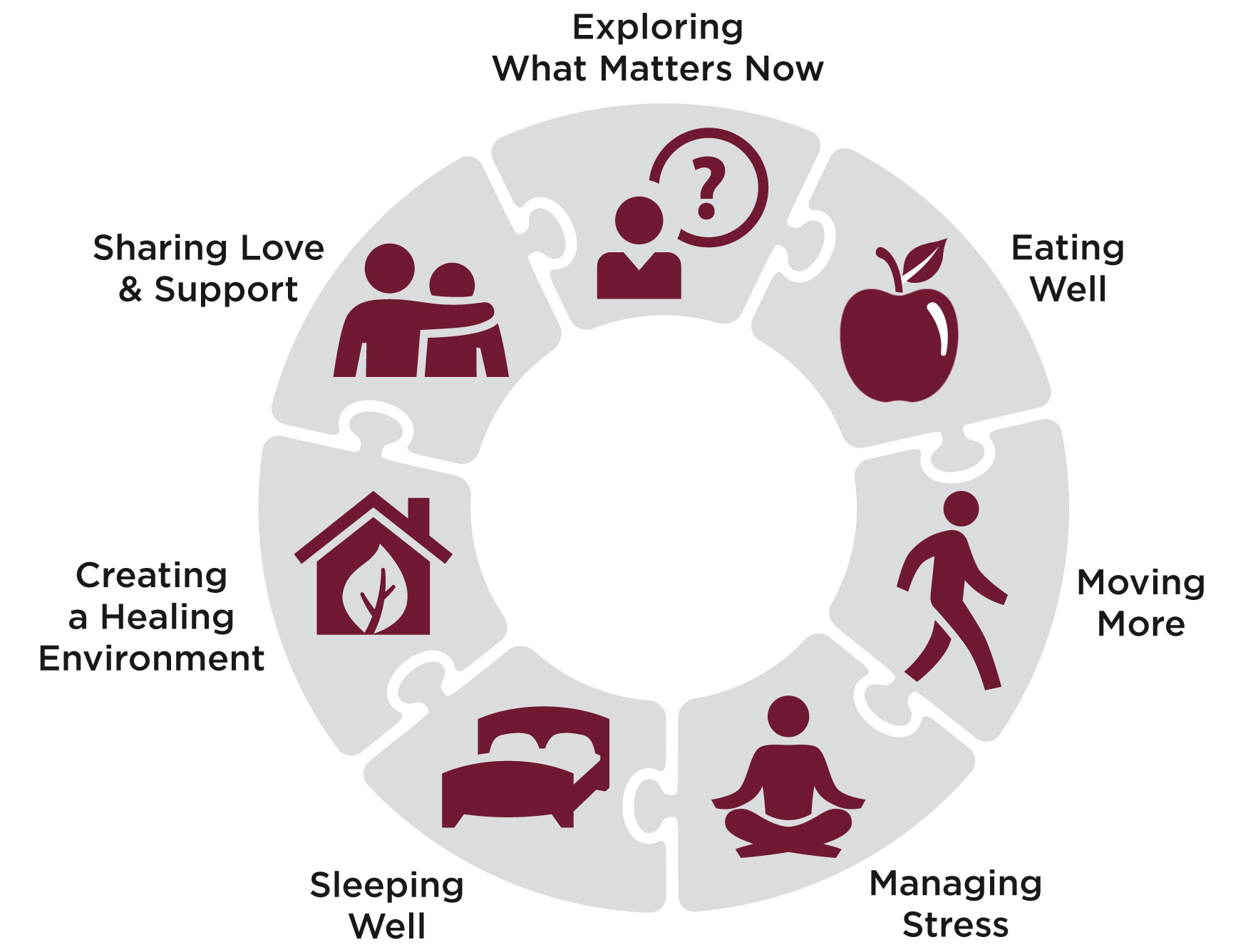 Any of the 7 Healing Practices are a good beginning. Eating Well and Moving More pack a powerful one-two punch in potentially improving treatment outcomes, enhancing quality of life and/or reducing risk of recurrence in colorectal cancer. Moreover, evidence shows that Managing Stress, Sleeping Well, Creating a Healing Environment, Sharing Love and Support and Exploring What Matters Now can help patients and survivors. Ultimately, let your intuition guide you in choosing where to start with these healing practices.
Any of the 7 Healing Practices are a good beginning. Eating Well and Moving More pack a powerful one-two punch in potentially improving treatment outcomes, enhancing quality of life and/or reducing risk of recurrence in colorectal cancer. Moreover, evidence shows that Managing Stress, Sleeping Well, Creating a Healing Environment, Sharing Love and Support and Exploring What Matters Now can help patients and survivors. Ultimately, let your intuition guide you in choosing where to start with these healing practices.
Bundling Practices Leads to Better ResultsPeople who followed the World Cancer Research Fund/American Institute of Cancer Research recommendations on diet, physical activity, and body fatness prior to a diagnosis of colorectal cancer showed better overall and cancer-specific survival after diagnosis. The more recommendations that were followed, the better the outcomes.25 A 2018 study of almost 1000 colorectal cancer survivors found a 42 percent reduction in death at five years for those who followed the American Cancer Society nutrition and physical activity guidelines most closely, compared to those who followed them least.26 |
Eating Well
Treating the Cancer
Ask for GuidanceA small study of colorectal cancer survivors in the United Kingdom found that most—more than 2/3—reported receiving no nutritional advice from their doctors and care teams.27 We have no reason to believe the situation is much better anywhere else. If your team doesn't provide guidance, ask your doctor for a referral to a dietician or nutritionist who specializes in counseling cancer patients and survivors. Even better, seek out an integrative healthcare provider (medical doctor, ostopathic doctor, naturopath, nurse or physician assistant who practices an integrative approach) if you'd like specific guidance about what to eat to improve your outcomes and manage side effects. |
Some food choices are associated with better or worse survival:
| Higher Survival | Lower Survival |
|---|---|
|
|
- The association is for colon cancer only; no association was found between processed meat intake and overall survival or disease-free survival for rectal cancer.32
Flax seeds, garlic, green tea and mushrooms and are among the plant foods most commonly used by oncology naturopaths for colorectal cancer.33
An observational study of patients with stage 3 colon cancer treated with surgery and adjuvant chemotherapy found a link between eating two or more weekly servings of tree nuts and improved disease-free survival and overall survival compared to no nut consumption.34
The ability of foods to influence inflammation may also impact survival. A diet with more anti-inflammatory potential improved overall survival among postmenopausal women diagnosed with colorectal cancer.35 Foods and food components with anti-inflammatory properties:
Managing Side Effects and Promoting Wellness
Higher intake of dietary magnesium is associated with less prevalent and less severe chemotherapy-induced peripheral neuropathy in colorectal cancer patients.36 Foods high in magnesium include these:37
- Almonds
- Black beans
- Cashews
- Dark chocolate
- Edamame beans
- Peanuts
- Pumpkin seeds
- Soy milk
- Spinach
- Whole-wheat bread or shredded wheat cereal
The Cancer.Net Editorial Board of the American Society of Clinical Oncology recommends a balanced diet that includes specific nutrients such as B vitamins (including B1 and B12, folic acid) and antioxidants (see Antioxidants and Cancer Outcomes below) to reduce pain from peripheral neuropathy. They also recommend reducing alcohol consumption.38
These foods are among those rich in B vitamins:39
- Eggs
- Leafy Greens
- Liver and Other Organ Meats
- Milk
- Salmon
Reducing Risk
Western dietary patterns—such as eating large amounts of processed meats and refined grains and low quantities of vegetables and fruits—has been associated with higher risk of tumor recurrence and mortality in colorectal cancer.40 More than 52,000 new colorectal cancer cases in the United States in 2015 were estimated to be associated with suboptimal diet among US adults.41 The American Institute for Cancer Research recommends a plant-based diet with a variety of fruits, vegetables, beans and whole grains to lower risk.42
As mentioned above, the Clinical Practice Guidelines Committee of the American Society of Colon and Rectal Surgeons recommends a balanced diet after curative treatment of colon and rectal cancer.43 One such balanced diet—the Mediterranean diet, and specifically its components olive oil, red wine, and tomatoes—is associated with clinically reduced cancer initiation and progression.44
Strong evidence shows these associations between food choices and risk of colorectal cancer or recurrence:
| Lower Risk | Higher Risk |
|---|---|
|
From many sources45
|
From many sources46
|
- Some evidence shows that fiber’s benefit may involve the gut microbiome.47
- Some evidence of reduced risk in men but not women48
A large study concluded that a moderate reduction in fat consumption did not reduce the risk of invasive colorectal cancer in postmenopausal women during more than eight years of follow-up.49
Studies and expert assessments have further concluded that these foods and dietary choices may also lower risk of developing colorectal cancer or recurrence of adenomas:50
- Foods containing vitamin C, found in peppers, parsley, kale, kiwis, broccoli, Brussels sprouts, lemons, strawberries, oranges and other foods
- Fish
- Non-starchy vegetables such as dark green and leafy vegetables
- Fruit
- Foods rich in folate (Healthline), such as legumes (lentils, peas and dried beans), asparagus, eggs, leafy greens and other foods
- Poultry, fish or legumes (dried beans, lentils and peas) instead of red meat
- Food with anti-inflammatory components (see the list above), including flavonols (such as quercetin) and vitamin D
Although early investigations suggested a protective effect of high intake of raw and/or cooked garlic against colorectal cancer,51 more recent analyses show no protective effect.52
Researchers evaluating the evidence across 80 meta-analyses of interventional and observational studies of colorectal cancer prevention found no evidence of a protective effect for tea, coffee, fish and soy products.53
Evidence shows that these foods may increase risk of colorectal cancer:
- Foods containing heme iron (red meat, chicken and fish) might increase the risk of colorectal cancer.54
- Foods with a high dietary inflammatory index:55
- Red and processed meats
- Refined carbohydrates
- Fried foods
- Sugar-sweetened beverages
- Margarine, shortening and lard
Antioxidants
Prospective randomized trials have not shown that antioxidant supplements prevent colorectal adenoma or carcinomas.56
B Vitamins
Higher dietary intakes of folate and riboflavin (vitamin B2) are associated with decreased risk.57 Eating foods higher in vitamin B12 was also associated with lower risk.58 and with an overall low-risk diet and lifestyle in a population at high risk for colorectal cancer.59 Good sources of these nutrients:60
| Folate | Riboflavin | Vitamin B12 |
|---|---|---|
|
|
|
a. See recommendations about eggs in the Commentary section below.
Unlike the B vitamins listed above, dietary intake of vitamin B6 shows mixed results:
- Reducing risk of colorectal cancer in some studies61
- Higher serum levels of vitamin B6 was associated with reduced risk in 50- to 69-year-old men.62
- A large meta-analysis found a slight decrease in colorectal cancer risk associated with the higher level of vitamin B6 intake. This decrease was not statistically significant, and dietary intake was not separated from supplement use.63
- Dietary B6 intake greatly increased risk of rectal cancer in women in one study.64
- A large study of US women aged 45 years or more found that dietary intakes of folate and vitamin B6 were associated with lower colorectal cancer risk only among women who were not taking supplements containing folate and vitamin B6.65
The takeaway with vitamin B6 is that its impact on colorectal cancer risk is uncertain. Benefits may apply only to specific groups or specific cancer types. To date, no compelling evidence suggests that the presence of vitamin B6 should be a priority in your dietary choices.
Calcium and Magnesium
- Higher intake of calcium in drinking water reduces risk of incidence and death from colon cancer.66
- Higher intake of dietary magnesium reduces risk of colorectal cancer, especially colon cancer.67
- With higher intake of magnesium or higher calcium-to-magnesium ratios, risk is also reduced for colorectal adenoma, but only in people with specific genes (genotypes).68
Fiber
Fiber feeds the friendly bacteria in your gut, and so is considered a prebiotic. Fiber is fermented by intestinal microorganisms into short-chain fatty acids, the most abundant of which is butyrate. Butyrate is necessary for normal metabolism but is not derived directly from food—it has to be created by bacteria fermenting fiber. Patients with colorectal cancer tend to have lower levels of butyrate-producing bacteria than other people.
Butyrate may be a reason that fiber is connected to colorectal cancer prevention. Butyrate is selectively transported into the lining of the colon, where it is used by normal colon cells for much of their energy needs. However, in cancer cells it accumulates in parts of the cell where its action is to suppress cell growth, induce cell death (apoptosis) and promote differentiation. In cell studies, butyrate inhibits colorectal cancer cell growth.69
Optimizing Your Terrain
Beneficial Foods
- Butyrate (from fiber, see above) is a potent anti-inflammatory. It lessens inflammation related to colitis in both rodents and humans.70
- Green tea consumption decreased fasting glucose and glycated hemoglobin (HbA1c) concentrations.71
- Cocoa is antioxidative and anti-inflammatory72
Foods to Avoid
- Diets high in cholesterol (WebMD) are linked to increased inflammation.73
See Eating Well.
Moving More
Treating the Cancer
Participating in regular physical activity reduces mortality:
- Reduced risk of colorectal cancer-specific mortality or overall mortality with any physical activity, with even lower risk with high levels of physical activity after diagnosis74
- People diagnosed with colorectal cancer who are at high levels of fitness had an 89 percent decreased risk of all-cause mortality75
- Each 15 MET-hours (metabolic equivalent task-hours) per week increase in physical activity after colorectal cancer diagnosis was associated with a 35 percent lower risk of colorectal cancer–specific mortality.76 Fifteen MET-hours per week is represented by any one of these activities:77
- 5 hours of general housecleaning, or
- 3½-4 hours of very brisk walking (4 miles per hour), or
- 3½-4 hours of moderate bicycling (10 to 12 miles per hour), or
- 2 to 2½ hours of singles tennis
Managing Side Effects and Promoting Wellness
Physical activity benefits some side effects and overall quality of life:
Quality of Life
- Survivors who met recommendations for physical activity reported higher health-related quality of life compared to those not meeting recommendations.78
- Physical activity directly related to improved physical function in older, long-term colorectal cancer survivors.79
- Physical activity was associated with higher total quality of life score, physical well-being, functional well-being, and other measures of quality of life.80
- Colorectal cancer survivors meeting Canadian public health exercise guidelines reported clinically and significantly better quality of life.81
- An exercise intervention among recently surgically resected colorectal cancer survivors found improved quality of life.82
- Previously active individuals who fail to reinitiate exercise after cancer treatment experience the lowest quality of life one to four years later compared to those who maintain activity, temporary relapsers and nonexercisers.83
Fatigue
- Colorectal cancer survivors meeting Canadian public health exercise guidelines reported clinically and significantly reduced fatigue.84
- Physical exercise has a positive effect on fatigue among cancer patients.85
Nausea
Sleep Disturbance
- While evidence shows that physical activity does promote better sleep87 sleep disturbance among colorectal cancer patients coming off first-line treatment was not improved by either an increase in exercise or a level of physical activity at or above American College of Sports Medicine's guidelines.88
Reducing Risk
The Clinical Practice Guidelines Committee of the American Society of Colon and Rectal Surgeons recommends regular exercise after curative treatment of colon and rectal cancer.89
Strong evidence shows that being physically active decreases the risk of colon cancer. Evidence is not conclusive regarding rectal cancer.90
- Those with high fitness showed a substantially decreased risk of incident colorectal cancer.91
- A large 2019 analysis found that engaging in 7.5 to 15 MET-hours per week (about 2.25 to 4.5 hours of brisk walking) was associated with a lower risk of colon cancer in men, as well as other types of cancer.92
See Moving More.
Managing Stress
Reducing Risk
Higher perceived stress is associated with increased risk of rectal cancer, but not colon cancer.93
See Managing Stress.
Sleeping Well
Treating the Cancer
Sleep duration and timing may impact survival:
- Short sleep duration (less than 5 hours per night) before diagnosis was associated with a 36 percent higher risk of all-cause mortality and a 54 percent increase in colorectal cancer mortality among colorectal cancer survivors.94
- Napping one hour or more per day before diagnosis was associated with significantly higher total and cardiovascular disease mortality but not colorectal cancer mortality.95 Colorectal cancer patients sleeping two or more hours during the day had a significantly increased risk of all-cause mortality compared to individuals with no daytime sleep.96 Keep in mind, however, that this does not mean that napping caused greater mortality. It's very possible that those who were already sicker needed to nap more, or that napping indicated disturbed nighttime sleep, and these underlying conditions contributed to greater mortality.
Circadian disruption—activity during sleeping hours and a lack of restful sleep—during chronomodulated chemotherapy is associated with shorter overall survival:
- The rest/activity rhythm was a strong predictor of both tumor response and survival in patients with metastatic colorectal cancer: patients with the poorest circadian rhythms had a five-fold higher risk of dying within two years than the patients with better circadian rhythms.97
- Patients with a disturbed circadian rhythm survived an average of 14.7 months compared to 22.3 months for patients with a robust circadian rhythm.98
- If a patient’s circadian rhythms are disrupted by chemotherapy, chronomodulated therapy may not be as effective. Chemotherapy-induced fatigue and weight loss—both of which are related to poor sleep quality—early in therapy may impair the benefits of chonomodulated therapy on survival and time to progression.99 The researchers suggest monitoring patients to detect early chemotherapy-induced circadian disruption. This will allow for adjustments in chronotherapy to improve safety and effectiveness.
Managing Side Effects and Promoting Wellness
Patients with restful sleep, as measured by clear distinctions between period of rest and of activity, had better quality of life and reported significantly less fatigue than patients with disrupted sleep. Disrupted circadian rhythms led to worse chemotherapy-related symptoms as well as patients’ perception of them.100
Sleep disturbance was associated with anxiety and fatigue among colorectal cancer survivors.101
Reducing Risk
The Clinical Practice Guidelines Committee of the American Society of Colon and Rectal Surgeons recommends regular sleep after curative treatment of colon and rectal cancer.102
- A 2014 review concluded that maintaining a regular and adequate daily amount of sleep reduces risk of colorectal cancer.103 However, long sleep duration—sleeping nine or more hours per night—is associated with an increased risk of colorectal cancer (but does not necessarily cause increased risk).104
- An extensive meta-analysis did not find an overall association between ever-exposure to night-shift work and the risk of colorectal cancer.105
Improving Sleep
Interventions recommended by integrative oncologist and BCCT advisor Dr. Keith Block to improve circadian rhythms and sleep for cancer patients:106
- Develop routine sleep habits.
- Get exposure to early morning bright light.
- Dispel incorrect notions about sleep.
- Keep your bedroom cool and dark.
- Supplement with melatonin.
- Consider cognitive-behavioral therapy for insomnia, which is effective for sleep problems in most cancers.
See Sleeping Well for more information.
Creating a Healing Environment
Reducing Risk
Several environmental exposures are associated with increased risk of colorectal cancer:107
- 1,1-dichloroethane (ToxFAQs™) used in industrial manufacturing of other chemicals, as a solvent for cleaning and degreasing, and in the manufacture of plastic wrap, adhesives, and synthetic fiber
- Alachlor (Beyond Pesticides), an herbicide
- Aromatic amines (Comprehensive Toxicology)
- Chlorination byproducts (Centers for Disease Control and Prevention)
- Ionizing radiation (World Health Organization)
- Nitrates in water (Centers for Disease Control and Prevention)
- Solvents (Centers for Disease Control and Prevention)
Chemicals formed during food processing—nitrosamines, heterocyclic amines and polycyclic aromatic hydrocarbons—may also be related to increased risk of colorectal cancer.108
See Creating a Healing Environment.
Sharing Love and Support
Managing Side Effects and Promoting Wellness
In a systematic review, emotional support and reassurance when trying to deal with fear of cancer recurrence featured as the most prominent supportive care need of colorectal cancer patients, regardless of clinical stage or phase of treatment.109
Evidence of the impact of social support on quality of life and symptoms:
- Lower levels of social support were correlated with higher levels of psychological distress among middle-aged colorectal cancer patients and their healthy spouses.110
- In patients undergoing surgery for colorectal cancer, greater social support, as well as improvements in insomnia and in physical, cognitive, and social functioning, improved anxiety and depression 12 months after surgery.111
- Greater perceived social support and resilience was associated with greater posttraumatic growth (positive change experienced as a result of the struggle with a major life crisis or a traumatic event) in colorectal cancer survivors with permanent intestinal ostomies.112
- Poorer quality of life outcomes (generic health-related quality of life, reduced well-being, anxiety, and depression) were significantly associated with lower levels of social support up to two years after surgery to cure colorectal cancer.113
Clinicians are encouraged to be “aware of situations that might necessitate intervention of other professionals, either medical or pastoral. Attention to psychosocial events is an integral part of a comprehensive oncologic program to facilitate patients and families to live in an atmosphere of peace and dignity.”114
Reducing Risk
Greater social support is related to greater engagement with colorectal cancer screening among Americans of African descent. Social support is also related to informed decision making about colorectal cancer screening among African American men in particular.115
Exploring What Matters Now
Managing Side Effects and Promoting Wellness
Making sense of the cancer experience was identified as a core theme affecting quality of life issues for colorectal cancer patients.116
See Exploring What Matters Now.
Beyond the 7 Healing Practices: Further Integrative Therapies
The Ultimate Guide to Cancer: DIY ResearchThis guide from Ralph Moss, PhD, BCCT advisor and leading chronicler of integrative cancer treatments, shows you how to use four of the main tools that doctors use to decide on the best cancer treatments. It will help you learn why some cancer treatments that look good in clinical trials may not work for “real world” patients. It will help you answer key questions that the doctor may be hesitant to answer in the detail you need to decide about treatment:
Also see The Moss Reports for comprehensive guidance on treating colorectal cancer. |
Conventional treatments are readily available. Complementary therapies can be useful to enhance conventional treatment effects, improve quality of life and possibly even extend life for those with colorectal cancer. Many complementary therapies―when chosen thoughtfully, reviewed with your oncology treatment team and used alongside conventional therapies—can become part of your integrative cancer care approach.
Therapies are grouped according to their effects:
- Treating the cancer
- Managing side effects and promoting wellness
- Reducing risk
- Optimizing Your Terrain
We present natural products in six groups:
- Good clinical evidence of efficacy & safety, easy access
- Good clinical evidence of efficacy & safety, limited access
- Limited clinical evidence of efficacy but good safety, used in leading integrative programs
- Limited clinical evidence of efficacy, or significant cautions, but potential significant benefit
- Especially promising preclinical or emerging clinical evidence of efficacy and safety
- Evidence of no efficacy or may be dangerous
Off-label, overlooked and novel cancer approaches (ONCAs) are grouped separately:
- Group A: Good clinical evidence of efficacy
- Group B: Limited clinical evidence of efficacy
- Group C: Promising preclinical evidence only
- Group D: Evidence of no efficacy or may be dangerous
Within each section, we list only groups containing applicable therapies.
Other integrative therapies and approaches are described but not categorized. See the full summaries as linked for more information on each of these therapies.
Treating the Cancer
Avoiding Drug Interactions during TreatmentPotentially life-threatening interactions between drugs are possible. For example, proton pump inhibitors (PPIs) can increase the risk for progression in colorectal cancer patients being treated with adjuvant CAPOX (capecitabine with oxaliplatin) or FOLFOX (leucovorin calcium [folinic acid], fluorouracil, and oxaliplatin). PPIs have a significant effect on both progression-free and overall survival. Experts conclude that “it is better to avoid PPIs during chemotherapy for colorectal and gastrointestinal tumors,” and avoid polypharmacy (the simultaneous use of multiple drugs to treat a single ailment or condition) whenever possible. From a report on a keynote speech from the ESMO 22nd World Congress on Gastrointestinal Cancer Virtual Experience in 2020.117 |
Working against cancer growth or spread, improving survival, or working with other treatments or therapies to improve their anticancer action
Conventional Treatments
Conventional treatments for colorectal cancer include these:
- Surgery (also see Surgery and Colorectal Cancer below)
- Radiofrequency ablation
- Cryosurgery
- Chemotherapy
- Radiation therapy
- Targeted therapy
- Immunotherapy
These treatments are explained on the National Cancer Institute website: Colorectal Cancer—Patient Version and Colorectal Cancer—Health Professional Version.
Newer conventional treatments and outcomes:
- Pressurised intraperitoneal aerosol chemotherapy (PIPAC) is a relatively new treatment for patients with peritoneal metastases. A 2019 review found an objective clinical response of 71–86 percent for colorectal cancer (median survival of 16 months) with PIPAC. Repeated PIPAC did not have a negative effect on quality of life.118
- Pulsed low-dose rate radiation therapy (PLDR-RT) delivers conventional radiation doses in pulses of small doses with intermittent pauses. A small study involved PLDR-RT for patients with rectal and other cancers of the pelvis. Patients had undergone radiation therapy to the pelvis previously. Twenty-three patients were treated with a curative intent and 15 were treated palliatively. At one year, 59 percent of patients treated for curative intent had a clinical, biochemical or radiographic response, and six of the 23 patients had no evidence of disease at their last follow-up. Among the patients treated palliatively, 61 percent had a clinical or radiographic response.119 This delivery also produces low rates of toxicity, along with reduced damage to noncancerous tissue and decreased repair of DNA damage in tumor cells.
Conventional treatments can be very expensive, and some treatments can cause long-lasting side effects.120 We encourage you to explore the benefits, risks and costs of all options.
Delaying Treatment
Some providers offer a “watch-and-wait” approach for select rectal cancer patients who have had a clinical complete response after neoadjuvant therapy. While this approach has resulted in excellent rectal preservation and pelvic tumor control, a 2019 study found it has also resulted in worse survival and a higher incidence of distant progression in patients with local regrowth compared to those without local regrowth.121 A review and meta-analysis in late 2020 confirmed that delaying colorectal cancer treatment by a month or more increases the risk of dying.122
Factors Influencing the Success of TreatmentCharacteristics of both healthcare providers and the patient can impact the likelihood of success in treatment. A surgeon’s or hospital’s frequency of performing high-risk surgeries can influence treatment outcomes. Surgeons and hospitals that do not perform at least a minimum number of these surgeries every year have a higher likelihood of errors, complications and even death. A 2019 review concluded that the minimum number of rectal cancer surgeries for competence was 16 for a hospital and six for each surgeon.123 Outcomes from all therapies and treatments can be influenced by a patient’s physical and psychosocial situation.124
|
More on Conventional Treatments
We recommend these resources to introduce you to the science of colorectal cancer and conventional therapies:
- National Cancer Institute:
- Cancer.net: Colorectal Cancer
Natural Products
Antioxidants and Cancer OutcomesSubstances that act as antioxidants can have both antitumor and tumor-promoting effects, depending on several factors:125
Many substances can serve as antioxidants and are abundant in these food sources:
Many of these individual antioxidants are also available as dietary supplements. Antioxidants have mixed effects on chemotherapy toxicity, but no trials have assessed long-term effects of antioxidant supplementation during chemotherapy on recurrence or survival. Mixed effects of antioxidants have been seen in reducing toxicity of radiotherapy, although not involving colorectal cancer patients. Observational studies in colorectal cancer patients have found that those taking self-prescribed multivitamins showed neither benefit nor harm regarding toxicity or survival.126 Antioxidants may reduce chemotherapy and radiotherapy toxicity, but they also can make these treatments less effective. The anticancer effects of radiotherapy and certain chemotherapy drugs, including alkylating agents, anthracyclines, podophyllin derivatives, platinum complexes and camptothecins, may come from producing reactive oxygen species and increasing cell death. A 2014 review concluded that accumulating evidence “does not support the widespread use of antioxidants in patients with cancer.”127 Antioxidants have shown little to no effect on reducing risk of colorectal cancer.128 Some evidence shows benefit in reducing recurrence: patients receiving an antioxidant compound of selenium, zinc, vitamin A, vitamin C and vitamin E were significantly less likely to have an adenoma recurrence.129 Use of tobacco and alcohol is an important consideration when considering antioxidant supplements. One analysis found that supplementation with antioxidants decreased the recurrence of colon adenomas among people who neither smoke nor drink alcohol, but use doubled the risk among participants who smoked and also drank more than one alcoholic drink per day.130 Evidence and cautions regarding eating foods rich in antioxidants are described in Eating Well above, while those related to supplements are listed in the Natural Products sections. |
Group 1: Good clinical evidence of efficacy & safety, easy access
These therapies may be widely used in integrative cancer protocols and traditional medical systems.
| Therapy | Notes |
|---|---|
| Medicinal mushrooms |
|
| Vitamin D |
|
Group 3: Limited clinical evidence of efficacy but good safety, used in leading integrative programs
| Therapy | Notes |
|---|---|
| Astragalus |
|
| Curcumin |
|
| Fermented wheat germ extract |
|
|
The effects of drinking tea are discussed above in Eating Well. |
|
| Melatonin |
|
| Mistletoe (European) |
|
|
Omega-3 fatty acid supplements The effects of omega-3s in your diet are discussed above in Eating Well. |
|
| Resveratrol |
Group 4: Potential significant benefit, but either limited clinical evidence of efficacy or significant cautions
May be used in leading integrative oncology programs. Therapies in this group may need more medical oversight and surveillance.
| Therapy | Notes |
|---|---|
|
Aged garlic extract (CAM Cancer) The effects of garlic in your diet are discussed above in Eating Well. |
|
| Combinations of therapies |
|
| L-carnosine (WebMD) |
|
| Vitamin B3 supplements |
|
| Vitamin C supplementation or intravenous use |
|
Other therapies with preclinical evidence only for treating the cancer |
Group 5: Especially promising preclinical or emerging clinical evidence of efficacy and safety
| Therapy | Notes |
|---|---|
| Arabinogalactan (WebMD) | |
| Grape seed extract | |
| Indole-3-carbinol supplements (About Herbs) |
|
| L-glycine (Healthline) |
|
| Probiotics |
|
Off-label, Overlooked or Novel Cancer Approaches (ONCAs)
These therapies have exciting potential and/or proven benefits. However, some carry higher risks of side effects, interactions with other treatments and other adverse medical events than other therapies we review. Cautions are noted with each therapy, and we strongly urge you to consult your doctor before using these therapies—even over-the-counter drugs—for cancer treatment. We also note whether a prescription is needed or if a therapy is not widely available.
Group A: Good clinical evidence of efficacy
May be used in integrative protocols and programs
| Therapy | Notes |
|---|---|
| Aspirin |
|
| Chronomodulated therapies |
|
| Metformin |
|
| Statins |
|
Group B: Limited clinical evidence of efficacy
May be used in integrative protocols and programs
| Therapy | Notes |
|---|---|
| Artemisinin derivatives and artesunate |
|
| Cimetidine (Tagamet) |
|
| Chloroquine (Medline Plus) |
|
| Copper chelation with tetrathiomolybdate (TM) and other substances |
|
| Nelfinavir (Virocept) (Medline Plus) | |
| Nonsteroidal anti-inflammatory drugs (NSAIDs) other than aspirin (MedicineNet) |
|
| Rapamycin (sirolimus) |
|
Group C: Promising preclinical evidence only
| Therapy | Notes |
|---|---|
| Bisphosphonates (Cancer Research UK), including clodronate (Canada) and zoledronic acid (Reclast, Zometa), |
|
Diets and Metabolic Therapies
Short-term fasting (noteworthy preclinical evidence)
- As effective as chemotherapy in delaying the progression of a wide range of cancers in animals331
- Reduced tumor progression in mice with complete fasts of one to two days or alternating fasting and non-fasting days332
- Synergistic effect with vitamin C in delaying tumor progression in mice with colorectal cancer with the KRAS gene mutation333
- Enhanced the effect of virus-mediated cell killing in colorectal cancer cells while protecting normal colon cells334
- Alternate-day fasting inhibited tumor growth in mice without causing weight loss.335
- Note cautions on the Intermittent Fasting page.
Manipulative and Body-Based Methods
Acupuncture and Electroacupuncture
- Reduced average tumor size and other indicators of cancer using nanoporous needles in animals (needles that have micro/nano-scale pores on their surface)336
Therapies Using Heat, Sound, Light or Cutting-edge Radiotherapy
- Local or regional hyperthermia:
- Improved overall survival time of patients with liver metastases from colorectal cancer compared to chemotherapy alone337
- "Excellent survival outcomes in optimally selected patients" with colorectal cancer who have peritoneal metastases treated with systemic chemotherapy, then cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (CRS-HIPEC). Both oxaliplatin and mitomycin C had comparable effectiveness when given in the intraperitoneal cavity. (Report on a presentation at the ESMO 22nd World Congress on Gastrointestinal Cancer)338
- Greater rates of complete response and regression of the primary tumor339
- No improved survival and an increased risk of adverse events in colorectal cancer patients when adding HIPEC to cytoreductive surgery compared with receiving cytoreductive surgery alone340
- Whole-body hyperthermia:
- Improved response to chemotherapy and potentially improved survival341
Managing Side Effects and Promoting Wellness
Inflammation and Side EffectsAs integrative oncologist and BCCT advisor Keith Block, MD, explains: Inflammation can bring on cachexia—the severe wasting syndrome common among patients with solid tumors—and, especially, metastases. Cachexia, which is particularly common in cancers of the pancreas, colon and lung, can lead to the rapid breakdown of muscle, including the heart muscle.342 Inflammation is associated with cachexia,343 as inflammatory cytokines cause reduced appetite and abnormal metabolism of proteins, fats and carbohydrates. All this leads to loss of muscle and weight.344 |
Side effects of the cancer and of treatments can dramatically impact your quality of life. A 2009 review summarizes: “Although issues and symptoms were most prominent during the first three years, long-term effects of treatment can persist and include fatigue, sleep difficulty, fear of recurrence, anxiety, depression, negative body image, sensory neuropathy, gastrointestinal problems, urinary incontinence, and sexual dysfunction.”345 Therapies that address side effects can greatly improve your well-being and improve life for you and your caregivers.
Conventional Treatments
Pulsed low-dose rate radiation therapy (PLDR-RT) delivers conventional radiation doses in pulses of small doses with intermittent pauses. A small study involved PLDR-RT for rectal and other cancers of the pelvis. Of the 50 percent of patients who reported pain at the local site before treatment, 68 percent reported an improvement in pain after PLDT-RT.346
Natural Products
Group 1: Good clinical evidence of efficacy & safety, easy access
These therapies may be widely used in integrative cancer protocols and traditional medical systems.
| Therapy | Notes |
|---|---|
| Astragalus |
|
| Curcumin |
|
|
The effects of ginger in your diet are discussed above in Eating Well. |
|
| L-glutamine, also known as glutamine |
|
| Melatonin |
|
|
Omega-3 fatty acid supplements The effects of omega-3s in your diet are discussed above in Eating Well. |
|
| Probiotics |
|
Group 2: Good clinical evidence of efficacy & safety, limited access
Some may require a prescription, for example.
| Therapy | Notes |
|---|---|
| Medical cannabis and cannabinoids |
|
Group 3: Limited clinical evidence of efficacy but good safety, used in leading integrative programs
| Therapy | Notes |
|---|---|
| Glutathione |
|
|
Magnesium (About Herbs) Evidence regarding magnesium in your diet is listed above in Eating Well. |
|
| Mistletoe (European) |
|
| N-acetylcysteine (About Herbs) |
|
Group 4: Potential significant benefit, but either limited clinical evidence of efficacy or significant cautions
May be used in leading integrative oncology programs. Therapies in this group may need more medical oversight and surveillance.
| Therapy | Notes |
|---|---|
| Combinations of therapies |
|
| Curcumin |
|
| Fermented wheat germ extract | |
| L-carnosine (WebMD) |
|
| Selenium supplements |
|
| Vitamin B supplements |
|
| Vitamin C (intravenous) |
|
| Vitamin E supplementation |
|
Group 5 Especially promising preclinical or emerging clinical evidence of efficacy and safety
| Therapy | Notes |
|---|---|
|
Aged garlic extract (CAM Cancer) The effects of garlic in your diet are discussed above in Eating Well. |
|
| Grape seed extract |
|
| L-glycine (Healthline) |
|
Off-label, Overlooked or Novel Cancer Approaches (ONCAs)
These therapies have exciting potential and/or proven benefits. However, some carry higher risks of side effects, interactions with other treatments and other adverse medical events than other therapies we review. Cautions are noted with each therapy, and we strongly urge you to consult your doctor before using these therapies—even over-the-counter drugs—for cancer treatment. We also note whether a prescription is needed or if a therapy is not widely available.
Group A: Good clinical evidence of efficacy
May be used in integrative protocols and programs
| Therapy | Notes |
|---|---|
| Chronomodulated therapies |
|
| Metformin |
|
Group B: Limited clinical evidence of efficacy
May be used in integrative protocols and programs
| Therapy | Notes |
|---|---|
| Aspirin (WebMD) |
|
| Bisphosphonates (Cancer Research UK) |
|
| Cimetidine (Tagamet HB) |
|
| Statins |
|
Diets and Metabolic Therapies
- Reduced chemotherapy-related fatigue, weakness, and gastrointestinal side effects while fasting without impairing the effect of chemotherapy460
- Increased protection against stressors including toxics in patients who fasted for 48 hours or longer around the time of platinum-based chemotherapy461
- Limited weight loss and toxicity to the heart and cardiovascular system related to chemotherapy462
- Reduced DNA damage in white blood cells (leukocytes) in patients who fasted for 48 hours or longer around the time of platinum-based chemotherapy463
- Noteworthy preclinical evidence:
- Protected mice against irinotecan side effects464
- Protected normal cells from the toxic effects of chemotherapy drugs while sensitizing cancer cells to the treatment465
- Reduced suppression of immune function and mortality caused by chemotherapy and promoted regenerative effects on stem cells in cell and animal studies466
- Note cautions on our Intermittent Fasting page.
- Used in the Block program for colorectal cancer467
For people having significant side effects—especially gastrointestinal—from chemotherapy, naturopathic oncologist and BCCT advisor Lise Alschuler recommends fasting for 48 hours, from after dinner on the day before chemotherapy, through the day of chemo and the day following. This can be a water fast (which includes coconut water and vegetable broths), or you can eat up to 600 calories per day of vegetable soup and/or low-carb vegetables. She stresses the importance of your being motivated to fast for success, and also that fasting during chemotherapy should be cleared with your treating oncologist. You should modify or stop the fast if you become dizzy or weak (in which case you can try adding boiled eggs or nuts), or if you feel worse than if you had eaten.
Mind-Body, Spiritual and Consciousness-changing Approaches
- Relaxation with guided imagery can reduce anxiety, pain and narcotic use following colorectal surgery and increase patient satisfaction.468
- More effects of guided imagery with cancer in general are described on our Guided Imagery page.
Manipulative and Body-Based Methods
Acupuncture and electroacupuncture
- Improved peripheral nerve symptoms and function, lowered incidence of chemotherapy-induced peripheral neuropathy, and reduced the need to for symptom mitigation in small studies469
- Reduced reported pain and toxicity to nerves from chemotherapy, and improved quality of life in an uncontrolled pilot study of ultrasound acupuncture;470 a related clinical trial is investigating the effectiveness and safety with colorectal cancer patients471
- Enhanced the effectiveness of ondansetron in reducing nausea, vomiting, abdominal distention and diarrhea, reduced length of hospital stay and improved wellness in patients receiving hyperthermic intraperitoneal chemotherapy after surgery472
Reducing Risk
Reducing the risk of developing cancer or the risk of recurrence
Risk Factors
These factors increase risk of colorectal cancer:473
- Inflammation
- Abnormal blood glucose (glycemia)
- Increasing age
- Family history of colorectal cancer
- Race, with African-Americans at increased risk
- History of abdominal radiation
- Diabetes mellitus and insulin resistance
- Moderate or severe famine before adulthood in women
- Metabolic syndrome, defined by having several of these conditions:474
- Increased blood pressure
- High blood sugar
- Excess body fat around the waist
- Abnormal cholesterol or triglyceride levels
Creating Healthy Habits: Lifestyle Associations
The role of the 7 Healing Practices in reducing risk is described above. Further lifestyle choices also relate to your risk of colorectal cancer:475
- Body fat/obesity, including high body mass index (BMI) early in life, especially in men. Risk increases 2 to 3 percent with each increased unit of BMI. Even among people considered of normal weight and not overweight (BMI < 25), increased body fat was associated with increased risk of colon cancer, but only in men).476 Obesity is also associated with worse cancer outcomes, such as higher risk of recurrence of the primary cancer or mortality.
- Drinking two or more alcoholic drinks daily increases risk of developing colorectal cancer, especially among men. Moderate alcohol consumption (2-3 drinks) increases risk 20 percent, and higher consumption may increase risk up to 50 percent.
- Smoking tobacco increases risk of colorectal and other cancers; risk increases with the amount of smoking, similar to alcohol consumption.
- Night shift work is correlated with a 30 percent or higher increased risk of colorectal cancer.
- Combination hormone-replacement therapy in women decreases risk, but must be weighed against other health risks associated with use. Colorectal cancers found in women taking hormone therapy after menopause may be at a more advanced stage.
Natural Products
Group 1: Good clinical evidence of efficacy & safety, easy access
These therapies may be widely used in integrative cancer protocols and traditional medical systems.
| Therapy | Notes |
|---|---|
|
Calcium supplements (About Herbs) Evidence regarding calcium in your diet is listed above in Eating Well. |
|
|
Magnesium supplements (About Herbs) Evidence regarding magnesium in your diet is listed above in Eating Well. |
|
|
|
|
Vitamin B supplements |
|
Group 3: Limited clinical evidence of efficacy but good safety, used in leading integrative programs
| Therapy | Notes |
|---|---|
| Combination therapies | |
| Curcumin |
|
|
The effects of drinking tea are discussed above in Eating Well. |
|
| Multivitamin supplements | |
|
Omega-3 fatty acid supplements The effects of omega-3s in your diet are discussed above in Eating Well. |
|
| Probiotics |
|
| Resveratrol | |
| Vitamin D |
|
| Vitamin E supplements |
|
Group 4: Potential significant benefit, but either limited clinical evidence of efficacy or significant cautions
May be used in leading integrative oncology programs. Therapies in this group may need more medical oversight and surveillance.
| Therapy | Notes |
|---|---|
| Fermented wheat germ extract |
|
| Selenium |
Group 5: Especially promising preclinical or emerging clinical evidence of efficacy and safety
Other therapies with preclinical evidence only for reducing risk
|
| Therapy | Notes |
|---|---|
| Astragalus and other saponins |
|
| Combinations of therapies |
|
|
The effects of ginger in your diet are discussed above in Eating Well. |
|
| Grape seed extract |
Group 6: Evidence of no efficacy or may be dangerous
| Therapy | Notes |
|---|---|
|
Aged garlic extract (CAM Cancer) The effects of garlic in your diet are discussed above in Eating Well. |
|
|
Beta-carotene supplements (About Herbs) The effects of foods containing beta-carotene are discussed above in Eating Well. |
Increased risk of colorectal adenoma and overall mortality in the general population562 |
| Folic acid (About Herbs) | No convincing evidence of reduced risk of colorectal cancer or adenomas in average-risk or high-risk populations; one randomized controlled trial found an increase in advanced adenomas with use563 |
Off-label, Overlooked or Novel Cancer Approaches (ONCAs)
Group A: Good clinical evidence of efficacy
May be used in integrative protocols and programs
| Therapy | Notes |
|---|---|
| Aspirin |
|
| Bisphosphonates (Cancer Research UK) |
|
| Metformin |
|
| Nonsteroidal anti-inflammatory drugs (NSAIDs) other than aspirin (MedicineNet) |
|
|
Thiazolidinediones (TZDs) (Diabetes.co.uk) Examples include pioglitazone (Actos) and rosiglitazone (Avandia) |
|
Group B: Limited clinical evidence of efficacy
May be used in integrative protocols and programs
| Therapy | Notes |
|---|---|
| Artesunate |
|
|
Optimizing Your Terrain
Cytokines, Inflammation and OutcomesCytokines are proteins with a complex relationship to your immune system and sleep cycles. If your circadian rhythm is disrupted by an external change in the light-dark cycle—such as by night-shift work or staying awake late at night—your immune cells produce a heightened inflammatory response driven in part by cytokine release.599 In patients with metastatic colorectal cancer, higher levels of inflammatory cytokines were linked to disrupted rest/activity circadian rhythms. Higher cytokine levels were associated with poorer response to chronochemotherapy (chemotherapy timed by circadian rhythms), poorer survival, increased fatigue and loss of appetite.600 Therapies that reduce inflammation and promote more typical sleep-activity rhythms may impact cytokine release and improve outcomes. |
Creating an environment within your body that does not support cancer development, growth or spread
See Body Terrain and the Tumor Microenvironment.
Natural Products
| Therapy | Notes |
|---|---|
|
Garlic cupplements, including aged garlic extract (CAM Cancer) The effects of garlic in your diet are discussed above in Eating Well. |
|
| Astragalus and other saponins | |
|
Combinations of therapies
|
|
| Curcumin |
|
| Fermented wheat germ extract | |
| Ginger |
|
| L-glutamine, also known as glutamine |
|
| Grape seed extract | |
|
The effects of drinking tea are discussed above in Eating Well. |
|
| L-carnosine (WebMD) | Antioxidant and anti-inflammatory653 |
| L-glycine (Healthline) |
|
|
The effects of omega-3s in your diet are discussed above in Eating Well. |
|
| Probiotics | |
| Turkey tail mushrooms or extracts | |
| Vitamin C |
|
| Vitamin E supplements |
|
Off-label, Overlooked or Novel Cancer Approaches (ONCAs)
| Therapy | Notes |
|---|---|
| Aspirin |
|
| Bisphosphonates (Cancer Research UK) (Clodronate liposomes) | |
| Cimetidine (Tagamet HB) |
|
| Copper chelation with tetrathiomolybdate (TM) and other substances | |
| Metformin | |
| Nonsteroidal anti-inflammatory drugs (NSAIDs) other than aspirin |
|
| Rapamycin (sirolimus) |
|
| Statins |
Other Therapies
Acupuncture and Electroacupuncture
- Electroacupuncture during laparoscopic radical rectectomy for rectal cancer decreased markers of inflammation after surgery.687
- Antioxidant and anti-inflammatory688
- Altered growth factors and metabolite levels, reducing the capability of cancer cells to adapt and survive;689 similar effects can be achieved with a fasting-mimicking diet (FMD)690
- Promoted cell self-clearing (autophagy); similar effects can be achieved with a fasting-mimicking diet (FMD)691
Your Microbiome and Colorectal Cancer
Antibiotic Use and Colorectal CancerAntibiotics can dramatically alter your microbiome. More frequent or oral antibiotic use was linked to a 17% increased risk of colon cancer but a reduced risk of rectal cancer (mostly among women) in a very large observational study.692 In a separate very large study, the increased risk was evident even with minimal use or with use 10 or more years prior to diagnosis, and risk was strongest with antibiotics with anti-anaerobic effects.693 “When asked about the difference between the apparent impact of antibiotic use on the risk of cancer in the colon when compared to the rectum, [senior author Cynthia] Sears commented, ‘We think these differences highlight the differences in biology and likely the microbiome between these two cancer sites. Hence we hypothesize that antibiotics impact disease at these sites differently.’”694 |
We know that lifestyle factors and your gut microbiome interact to influence the development and progression of colorectal cancer. We are not yet clear on exactly how this plays out in people or what we can do to manipulate the microbiome favorably. We know that diet influences the microbial community in the gut. Researchers think the interaction between diet and gut microbiota influences colorectal cancer development by changing your metabolism and immune system.695 Evidence supports these assertions:
- A high-fat diet is bad news for gut health, as it produces secondary bile acids. These acids change the microbiome, resulting in increased oxidation and inflammation that damage colon cells.696
- Beneficial bacteria in the gut are needed to process and create essential nutrients by fermenting dietary fiber and producing butyrate. These microbial processes provide energy to colon cells and promote protective immune system effects. Adequate dietary fiber is thus essential for a healthy interplay between the gut microbiome, colon cells and immunity.697 Lower levels of butyrate-producing bacteria are associated with the presence of colorectal cancer.698
- Impacts of a healthy microbiome with colorectal cancer include these:699
- A healthy gut microbiome appears to support the anticancer action of the chemotherapy drug oxaliplatin.
- Bacteria in the genus Bifidobacterium are crucial to optimizing the anticancer action of ligand 1 drugs (PD 1 checkpoint inhibitors), which activate the immune system to attack tumors.
- Gut microbes can prevent reactivation of drug metabolites that can damage the intestines and cause diarrhea related to drugs such as camptothecin.
- Microbial species in the intestines can impact inflammation.
People with colorectal cancer have less diverse gut bacteria, with reduced levels of Bifidobacterium, Clostridium, Faecalibacterium and Roseburia, for instance. Harmful species including Escherichia coli, E. faecalis, F. nucleatum, and Streptococcus gallolyticus also tend to be present in colorectal cancer patients.700 For example, enterotoxigenic Bacteroides fragilis [ETBF], which produces toxins in the digestive tract, is associated with a greater number of early-stage carcinogenic lesions and increased risk of colorectal cancer.701
Probiotics, Prebiotics and Synbiotics
Probiotics are living microorganisms (bacteria and some yeasts) that can provide health benefits that go beyond basic nutrition, such as supporting gut and immune health and keeping the gut microbiota in balance. Examples of probiotic foods are yogurt, kefir, sauerkraut, tempeh and kimchi. Probiotics must be consumed in sufficient numbers to be effective.
Prebiotics are dietary fibers that feed the friendly bacteria in your gut. Most prebiotics are soluble fiber substances like inulin, found in foods like bananas, onions, jerusalem artichokes, jicama, garlic and others, plus chicory root. Your helpful bacteria turn inulin and other fibers into energy for the colon cells and create protective immunity. Inulin is increasingly being added to a number of processed foods and probiotic supplements.
Synbiotics contain prebiotics and probiotics together.
Use of pre- and probiotics can reduce some symptoms and side effects of cancer treatments and can improve the gut microbiome and impact inflammation as described above.
See our Probiotics summary for more information.
Surgery and Colorectal Cancer
Key Points: Surgery and Colorectal Cancer
|
Colorectal cancer treatment often includes surgery. The surgery may provide long-term benefit regarding cancer outcomes, but risks and complications are also relatively commonplace. We provide a brief overview of issues and integrative approaches surrounding colorectal cancer surgery. General information about surgery with cancer is available on our Integrative Approaches to Surgery page.
Clinical Practice Guidelines
For Healthcare Professionals: Enhanced Recovery after Surgery (ERAS)ERAS is an approach focusing on counselling before surgery, optimizing nutrition, standardizing approaches to pain relief and getting you (the patient) moving and on your feet following surgery. It draws from several modalities, such as nutrition, medication, movement and counseling.702 In patients undergoing extensive pelvic dissection, ERAS can improve recovery, reduce the rate of complications and reduce the length of hospital stay following surgery. ERAS also provides early warning for later complications.703 See a discussion of ERAS protocols and outcomes: Enhanced recovery after rectal surgery: what we have learned so far and Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) group recommendations. ERAS includes returning to eating by mouth after surgery as soon as practical, with several benefits:704
Being informed and engaged is key to optimal nutrition following surgery. Optimal nutrition also improves your body terrain factors:
|
Guidelines for patients from the Enhanced Recovery After Surgery (ERAS®) Society:707
- Recommendations before hospital admission:
- Stop smoking at least four weeks before surgery to reduce problems with breathing and wound healing
- Engage in a prehab activity program (see below) to promote quicker recovery of function and fewer complications, especially if you are less fit
- Recommendations before surgery:
- Avoid sedatives such as benzodiazepines if possible; taper a withdrawal if needed.708 Also, see Integrative Approaches and Surgery for a list of supplements to stop taking before surgery.
- Recommendations following surgery:
- When you are allowed to eat, choose healthier foods from the menu. See Integrative Approaches and Surgery for examples of healthy eating when recovering from surgery.
- If prescribed, use oral nutritional supplements from the day of surgery or as directed by your doctor.
- Move as much as comfortable, including getting on your feet as soon as you can.
The American Society of Colon and Rectal Surgeons and Society of American Gastrointestinal and Endoscopic Surgeons provide guidelines for the surgical team: Clinical practice guidelines for enhanced recovery after colon and rectal surgery from the American Society of Colon and Rectal Surgeons and Society of American Gastrointestinal and Endoscopic Surgeons.709
Prehab and Surgical Outcomes
Prehabilitation (prehab), “the process of enhancing physical fitness before an operation to enable the patient to withstand the stress of surgery,” can reduce several risk factors for surgical complications, including malnutrition, anxiety and depression, and may also help to manage uncontrolled conditions or comorbidities, including glycemia, diabetes, hypertension and anemia.710
Prehab may include exercise training, counseling and oversight regarding nutrition, and strategies for coping with anxiety and distress. See information about nutrition in the Nutrition and Surgery section of our Integrative Approaches and Surgery page. Information about managing anxiety before surgery is in the Managing Anxiety before Surgery section of that page.
Nutritional guidelines for patients undergoing surgery for colorectal cancer:711
- Meet your energy requirements: One in four colorectal cancer patients has elevated metabolism (hypermetabolism), even those with good physical status. Hypermetabolism is linked to negative energy balance, weight loss, systemic inflammation and decreased ability to function in daily activities. Common formulas for determining energy requirements are not accurate in this situation. Work with your care team to use indirect calorimetry with adjustments for additional exercise and physical activity, which is more helpful.
- A high-protein diet, modified for those with kidney disease
- Meals should be balanced in this ratio
- Two servings of starches
- One of high-protein sources
- Two of vegetables
- Follow basic healthy dietary suggestions before surgery. See Eating Well, following further recommendations from your healthcare providers for your specific condition.
Surgical Factors Associated with Increased Recurrence Risk
Even though surgery is a routine treatment for solid tumors, surgery itself can promote the development of metastasis by releasing tumor cells into circulation, suppressing important immune defenses such as your cellular immune system and and promoting the development of blood vessels to supply tumors (angiogenesis).712
Type of Surgery: Open or Laparoscopic
The type of surgery—whether open surgery or laparoscopic surgery—has a great impact on the resulting inflammation—greater than the choice of anesthetic and pain management techniques (epidural versus intravenous analgesia).
Surgery initiates a local inflammatory response, starting with the incision, which the body interprets as a wound. Circulating tumor cells are drawn to wounds, infection sites and tissue trauma, setting up a microenvironment in distant organs conducive to the survival and growth of tumor cells. This is called a premetastatic niche. In addition, systemic inflammation—such as in metabolic syndrome, chronic stress response or chronic insomnia—also creates a microenvironment supportive to tumors.
The more extensive the surgery, the greater your inflammatory response. More extensive surgery could tip the stress-inflammatory response in the direction of metastasis even when the primary tumor is successfully removed. The wound-healing process can release immune system chemicals known to promote tumor growth.713 In fact, abdominal/pelvic surgery is associated with metastasis across the peritoneal cavity.714
It would seem that laparoscopic surgery reduces this potential. However, a small study in Europe found no significant difference in recurrence or survival between open and laparoscopic surgery in patients undergoing surgery with chemo-irradiated rectum tumors. The length of the follow-up period was not specified.715 Much more evidence is needed.
Surgical Conditions
Mild low body temperature (hypothermia) worsens the suppression of your immune response from abdominal surgery.716 Hypothermia may impair your immune system’s ability to stop infection and kill cancer cells. Maintaining your body temperature during surgery will reduce your risk of immune suppression.
Use of blood transfusion products can cause suppression of your immune response and increase your risk of recurrence.717 Blood transfusion using your own blood (autologous transfusion) may reduce your risk of recurrence.718
Patient Condition at the Time of Surgery
Your stress level and other characteristics around the time of surgery can affect your immune system and may increase your risk of recurrence:
- Your surgical stress response subdues your immune system’s ability to stop infection and kill cancer cells, increasing the likelihood that cancer cells will travel and lead to metastasis.719
- Your stress level can act to suppress your immune system separate from your surgical stress response. Higher levels of stress are linked to greater suppression of the immune system after surgery, including natural killer cells and the response of antitumor T cells.720
- Your physical condition: lower fitness for surgery—such as measured by a high Physiological and Operative Severity Score for the Enumeration of Mortality and Morbidity (POSSUM)—and a high systemic inflammatory response before surgery predict early disease recurrence after a potentially curative resection for colorectal cancer.721
- Your mood (anxiety and/or depression) can depress your immunity.722
Reducing Factors around the Time of Surgery that Increase Recurrence Risk
Delaying Surgery and SurvivalDelaying surgery may lead to poorer survival, according to a systematic review. Conclusions from the review:723
|
Combined use of the beta blocker propranolol and the anti-inflammatory etodolac for five days before surgery has been used safely to reduce metastases and mortality. However, this combination may not be safe in patients with asthma, cardiovascular disease, diabetes, bleeding risk, GI ulcers or low blood pressure.724
Taking precautions to prevent blood clots, neutrophil extracellular traps (NETs) and low oxygen levels (hypoxia) may reduce recurrence after surgery.725
See these pages for suggestions for managing stress or anxiety before and after surgery:
Surgical Complications and Infections
Surgical ComplicationsColorectal cancer surgery can involve several possible complications:726 During surgery:
After surgery:
|
Infections and complications of surgery not only make recovery more difficult, they may impact your cancer outcomes and even your survival (see sidebar).727
Colorectal surgery is invasive and disrupts the equilibrium of your gut microbiome—the microbes in your gut. A microbial imbalance can impair the function of your local immune response, promote systemic inflammation, and potentially lead to infection following surgery.728 Perhaps due to the large number of bacteria present in the colon and rectum, the number of surgical site infections in patients undergoing colorectal surgery is high—up to 26 percent.
Complications can reduce survival through several routes:
- Infection increases inflammation, which is associated with increased risk of local recurrence and cancer spread.
- Complications can delay chemotherapy treatment, which may lead to poorer outcomes.729
- Complications of surgery—especially anastomotic leaks (where colon sections are joined)—can lead to longer hospital stays and increased risk for hospital-acquired complications, as well as increased risks of readmission, of reoperations and of mortality.730
- Complications following surgery that decrease survival and increase recurrence risk:731
- Anastomotic leakage
- Pneumonia
- Bowel obstruction/ileus
- Infection at the surgical site
- Postoperative bleeding
- Urinary tract infection
- Fistula (an abnormal connection between two body parts)
Factors Increasing Risk of Infection and Other Complications
| Can Be Influenced or Controlled | Cannot Be Influenced |
|---|---|
|
|
| Factors Not Increasing Risk | |
|
- Obesity, smoking, glycemia, hypertension, diabetes, anemia-compromised immunity or inflammation, and patient age 65 or older are each linked to increased wound complications following surgery.732 In elective surgery in overweight patients, weight loss before surgery is recommended, as is correction of anemia with iron, vitamin B12 and folate supplementation as needed, such as for pernicious anemia.733
- Malnutrition is linked to a greater chance of surgical complications, longer hospital stay, less tolerance of other cancer treatments, higher risk of death and higher health-care costs.734
- Surgeons with more experience as well as hospitals with higher numbers of colorectal surgery patients are associated with fewer complications and, in some studies, lower risk of recurrence and higher survival.735
- Anxiety and depression before surgery negatively affect wound healing, your risks of infection and a longer hospital stay, and your ability to adhere to your medical treatment plan.736
- A 2016 meta-analysis found that radiation therapy before radical rectal cancer surgery didn’t increase risk of wound complications.737 However, some evidence shows increased risk of infection and other complications such as anastomotic leakage with chemoradiotherapy before surgery.738
- Men are at higher risk than women of anastomotic leaks with rectal cancer surgery.739
- The current standard to reduce infection risk is called mechanical bowel preparation (MBP, giving oral medicine to clear feces from the intestines) plus prophylactic antibiotics. Some research has suggested that MBP doesn’t improve infection outcomes and may cause greater harm because it is often poorly tolerated.740
- Low skeletal muscle mass and density were associated with longer hospital stays and higher risks of postsurgical complications, and both short-term and long-term mortality.741
Preventing Surgical Complications
Reducing the risk of complications: what you and your surgeon can do
Anastomotic LeaksAnastomotic leaks—occurring at the place where colon sections are joined after a section is removed— can lead to other problems such as longer hospital stays; higher risks of readmission, reoperations or mortality; and a worse quality of life. Patients who have anastomotic leaks following cancer operations also have a higher risk of distant recurrence and long delays in receiving indicated adjuvant (supplemental) chemotherapy. Recognized or proposed risk factors include these (also see the discussion below of pain control and surgical outcomes):742
Interventions for the surgical team to reduce the incidence of anastomotic leaks:743
Interventions for patients:
|
What You Can Do
- First, find out how much time you can take before surgery to develop a plan and prepare for surgery.
- Preparing your body
- Discuss which risk factors you can improve before surgery and come up with a plan of actions to take. Actions may include controlling hypertension, stress, hyperglycemia and other conditions, or stopping smoking
- Consider incorporating stress management practices in the weeks leading up to surgery. Many patients find imagery practices specific to preparing for surgery to be helpful. See Managing Stress, Stress, Mind-Body Approaches and Guided Imagery.
- In addition to effective stress management practices, use emotional support, counselling and pre-surgery medication as appropriate to help reduce preoperative psychological stress.
- If you typically clean an animal litter box or bird cage, find someone else to clean it before and for several weeks after your surgery.
- Optimizing your surgical context:
- Check postoperative infection rates for the hospital where your surgery will be performed at Medicare.gov—Hospital Compare. While individual surgeon complication rates are available for many types of surgery, they are not published for breast surgery. Having said that, infection rates tend to be higher on average with less-experienced surgeons (a pretty good rule of thumb for having good experience is to consider surgeons who have done at least four per month of your specific type of surgery for five years).
- Inform your surgical team of any supplements, herbs or other therapies you’re using prior to surgery.
- If you have financial or social barriers to good pre- and postsurgical care, ask to be referred to an oncology social worker or oncology navigator for assistance.
- Schedule your surgery as an ambulatory procedure rather than as an inpatient hospital stay, if possible.
- Discuss your options for anesthesia, post-surgical pain control (see more about ERAS protocols above) and the steps in the column at right with your surgical team at the pre-op visit.
- Immediately before surgery:
- Avoid presurgical dehydration.
- See if you can postpone surgery if you develop a cold, flu, pneumonia or other infection shortly before scheduled surgery.
- After surgery
- Before leaving the hospital, be sure you (and anyone who will be assisting you at home) fully understand and follow all wound care instructions carefully. Call your physician immediately if you show any signs of infection—an increase of redness, swelling, pain or discharge from your wound.
- Avoid contact with soil for two or more weeks after surgery.
- Consult an integrative physician or licensed naturopath (preferably one who is certified in oncology) to recommend approaches to maintain healthy immune function to improve your wound healing and reduce your risk of infection.
- In the weeks following your surgery, if you need a medical procedure that may introduce bacteria to the body, check with your surgeon about using antibiotics to prevent infection.
What Your Surgeon Can Do
- Assess all choices and optimize risk factors, including patient characteristics and their status of adjuvant therapy, such as radiotherapy and chemotherapy.
- Because half of infections occur more than 30 days after a procedure, implement a plan for follow-up care, including appointments and phone calls.
- Reduce suppression of the immune system induced by surgery and anesthesia:744
- Use regional anesthesia and IV propofol as the primary anesthetic when possible
- Provide adequate pain control throughout the surgical experience while minimizing the use of opioids such as morphine, oxycodone or codeine.
- Avoid opioids during or after surgery by using an intravenous propacetamol and anti-inflammatories such as ketorolac while in the hospital and then using oral anti-inflammatories such as ibuprofen or naproxen after discharge.
- Avoid hypothermia by maintaining core body temperature devices such as fluid warmers and external body warmers.
- Remove catheters and drains as soon as possible.
- Use antibiotic prophylaxis.
HA/CMC film adhesion barrier: A hyaluronic acid/carboxymethylcellulose (HA/CMC) film adhesion barrier can reduce adhesion formation, but a multicenter study found its use increased the risk of total adverse events and serious adverse events including excess body heat (hyperthermia), abscess in the pelvic area or incision site, urinary tract infection, urinary retention and ileus (bowel or intestinal blockage or paralysis).745
Infection and Treatment Outcomes
Infection may delay cancer treatments such as chemotherapy or radiation, leading to less effective treatment and worse outcomes, including recurrence.746
Some evidence shows that radiochemotherapy before surgery for rectal cancer may increase risk of infection and other complications such as anastomotic leakage.747 However, a 2016 meta-analysis found that radiation therapy alone before radical rectal cancer surgery didn’t increase risk of short-term wound complications,748 although side effects and a decreased quality of life may prolong recovery from surgery.749 Because treatment decreases the risk of local recurrence (but without changing cancer survival outcomes or your risk of distant metastasis), both risks and benefits need to be considered with your oncology team.750
Preventing Infection
Laparoscopic surgery: Fewer wound-related complications, including infections and fever, were seen with laparoscopic surgery compared to open surgery in patients with chemo-irradiated rectum tumors. The need for transfusion was also lower with laparoscopic surgery.751
Taurolidine: Non-metastatic colon cancer patients undergoing surgery receiving taurolidine (ScienceDirect) showed reduced inflammation, lower risk of surgical site infection and possibly lower rates of recurrence two years after surgery.752
Antibiotics: Systemic ultra-short and short-term antibiotic preventive treatment (prophylaxis) before and during surgery reduces the risk of postsurgical infection. Some studies suggest giving both oral and intravenous (IV) antibiotics for greater effect. Oral, non-absorbable antibiotics may reduce risk not only of surgical site infection but also of anastomotic leak.753
However, prolonged antibiotic use, such as what might be required if an infection or anastomotic leak develops following surgery, may impair the function of your immune and neuroendocrine systems, increasing your risk of future infection and/or recurrence. Minimizing your risk of infection and thus reducing the need for prolonged use of antibiotics is important.
Prebiotics or probiotics: Some studies conclude that prebiotic or probiotic use around the time of surgery may reduce infections following surgery and help maintain the intestinal mucosal barrier, while other studies have shown no effect.754
Early mobilization—patient movement as much and as often as tolerable, including getting out of bed and walking—reduces the risk of pneumonia as well as surgery complications not related to infection, such as deep vein thrombosis (DVT), muscle loss and insulin resistance.755
A 2018 review concluded that these measures reduce surgical site infection after rectal surgery:756
- Antibiotic prophylaxis (see below)
- Preventing low body temperature (hypothermia)
- Hair removal
- Preventing high levels of blood glucose (hyperglycemia)
Other proposed interventions:
- No fluid overload
- Skin preparation with chlorhexidine
- Double gloving or change of gloves and gowns before closing the fascia
- Lavage of subcutaneous tissue
- Silver dressing
Actions if You Develop an Infection
- Report symptoms of infection immediately to your surgeon and begin treatment promptly. If antibiotics are prescribed, take as directed.
- Eat well to maintain a healthy nutritional state. Consider consulting a board-certified oncology dietician for specific dietary recommendations.
- If antibiotics are prescribed, eat well and follow other practices to restore a healthy microorganism balance. See Eating Well, Mediterranean Diet and Your Microbiome.
- Consider consulting an integrative oncology specialist about additional measures to clear infection, help wound healing, control inflammation and minimize tissue scarring (fibrosis) from surgical wounds and/or from radiation therapy.
Pain Control
Sufficient pain control following surgery is essential to improve the quality of convalescence and speed up recovery.757 However, pain control methods vary considerably in their impact on surgery and cancer outcomes. Wise use of therapies to manage pain is extremely important to optimize both surgical and cancer outcomes.
Effectiveness of Pain Control Approaches
Drug-based Pain Management
- Opioid-based intravenous patient controlled analgesia: Compared to epidural analgesia in laparoscopic surgery, opioid-based intravenous patient-controlled analgesia (IV PCA) using fentanyl showed comparable pain control, faster return of bowel function, fewer overall complications, and shorter hospital stays, plus less need of drugs to maintain blood pressure.758
- Continuous surgical wound infiltration with local anesthetics used after laparoscopic colorectal surgery reported similar pain control efficacy as opioid-based IV PCA (above) in at least some patients.759
- Thoracic epidural analgesia (TEA) was more effective than IV-PCA (see above) after open colorectal cancer surgery, with a better bowel function, dietary intake, patient satisfaction and early mobilization in a small trial.760
- Quadratus lumborum block (QLB) was more effective analgesia following surgery than the transversus abdominis plane block.761
- Transabdominus plane (TAP) blocks for anesthesia as part of an enhanced recovery program with laparoscopic and robotic-assisted colorectal cancer surgery reduced the length of hospital stay, use of narcotics following surgery and the time until the patient was walking and resumed bowel function.762
- A small pilot study investigated a multimodal pain management protocol (administered after induction anesthesia) in patients undergoing a laparoscopic resection of colorectal cancer. The protocol used a bilateral TAP block and local abdominal cavity infiltration with long-acting local anesthetic liposomal bupivacaine. Patients on this protocol required fewer opioids during surgery, had shorter stays in the post-anesthesia care unit (PACU), less pain following surgery, less use of narcotics and a shorter hospital stay compared to a group that received no block or local wound infiltration.763
- The COX 2 selective inhibitor parecoxib—a non‐steroidal anti‐inflammatory drug—before surgical incision (compared to after incision) in colorectal cancer surgery reduced morphine use following surgery without affecting morphine-related side effects. Use before incision also reduced markers of inflammation.764
Non-drug Pain Management
- Acupuncture reduced the need for general anesthesia during rectal cancer surgery.765
- Transcutaneous electrical acupoint stimulation combined with transversus abdominis plane block (above): patients reported lower pain and lower opioid use following surgery than those receiving neither therapy.766
Also see our Integrative Approaches and Surgery page for further research on pain management in surgery for more cancer types and other settings.
Impact of Pain Control Methods on Surgical Outcomes
Non‐steroidal Anti‐inflammatory Drugs (NSAIDs)
Use of non‐steroidal anti‐inflammatory drugs (NSAIDs), and especially non‐selective NSAIDs, following surgery may increase your risk of anastomotic leakage. Non-selective NSAIDs include diclofenac, diflunisal, etodolac, fenoprofen, flurbiprofen, ibuprofen, indomethacin, and ketoprofen.767 Diclofenac or celecoxib use was especially related to increased risk, as were higher doses of NSAIDs and starting use less than 48 hours after surgery.768
Acupuncture and Electroacupuncture
Use of acupuncture showed these benefits:
- Reduced time to first bowel sounds, first flatus and first defecation following surgery for colorectal cancer769
- Shorter fasting and time to peritoneal drainage tube withdrawal770
- Shorter hospital stay, shorter time to first flatus and shorter time to defecation among patients receiving both acupuncture and simo decoction (a traditional Chinese medicine) for five days following colorectal cancer resection771
Electroacupuncture impacts:
- Following laparoscopic surgery:
- Reduced duration of inability of the intestines to contract normally, which can lead to intestinal blockage blockage (ileus)772
- Reduced time to start walking (mobility)773
- Reduced use of pain relievers following laparoscopic surgery for colorectal cancer774
- Quicker recovery of gastrointestinal function with electroacupunture administered three times: one day and 30 minutes before surgery and one day after surgery; no improvement was reported with one or two electroacupuncture administrations775
Transcutaneous electrical acupoint stimulation (TEAS) impacts:
Movement
A behavioral intervention—moving as soon as possible after surgery—reduced discomfort and the length of stay in the hospital.778
Opioids, Sedatives and Antidepressants
Adults undergoing colorectal resection who had used opioids, sedatives or antidepressants before surgery had higher rates of these outcomes compared to non-users:
- Ostomy creation
- Contaminated wound classification
- Prolonged operation time
- Transfusion following surgery
- Intra-abdominal infection
- Respiratory failure
- Longer hospital stays
- Increased 30-day morbidity and mortality
These patients also had lower fitness scores and more respiratory health issues than other patients.779
Impact of Pain Control Methods on Cancer Outcomes
Some approaches to managing pain may increase risks of suppressing your immune system and of cancer growth or recurrence.
Increased Risk of Immune Suppression and Possible Cancer Growth, Recurrence or Metastasis
General anesthesia: A small study of patients undergoing elective orthopedic surgery found a significant decrease of immune function using general anesthesia with fentanyl, thiopental and isoflurane.780
Regional anesthesia is favorable to general anesthesia―or even in addition to general anesthesia―for reducing inflammation, recurrence and metastasis in preliminary evidence.781
Volatile anesthetics: halothane, isoflurane, desflurane, and sevoflurane are volatile inhaled anesthetics that suppress the immune system and play a role in promoting cancer growth, perhaps through several pathways.782
Mixed Results
Opioids: The relationship of opioid drugs and cancer outcomes is difficult to separate from the effects of pain. Some evidence shows that opioid drugs—including morphine and tramadol—suppress immune responses and can promote tumor progression. However, cell studies have found that morphine both promoted and reduced processes of cell death (apoptosis). A 2014 review concluded that “further work is required to elucidate the possible impacts of morphine in cancer patients.”783
Preliminary evidence shows that some opioids may be used for short periods without increasing risk of cancer mortality:
- A study found no differences in overall survival or disease-free survival at five years when comparing outcomes of using epidural, spinal block, or a morphine patient-controlled analgesia (PCA) for primary pain relief following surgery.784
- A small study compared the opioid fentanyl used as intravenous patient-controlled analgesia (IV PCA) to a regimen of local anesthetic wound infiltration-based analgesic and tramadol. “Rescue” analgesics were used: pethidine for the opioid group and ketorolac or propacetamol for the group receiving local anesthetic. The two approaches were comparable regarding immune function (natural killer cell cytotoxicity) and complications following surgery and recurrence or metastasis within one year after surgery.785
- Tramadol shows protective effects on immune function and reduced risks of recurrence and metastasis.786
- One study found use of opioids vs. use of local anesthetic did not affect cancer recurrence or metastasis for one year following surgery.787
Given that opiods may disrupt immune responses and function, preventing immune disruption may be warranted with use.
- Pretreatment with immunotherapy such as interferon may reduce some of the negative effects of opioids on your immune response, as suggested in animal studies.788
- If opioids are indicated, lower doses may disrupt your immune system function less than larger doses.789
- Substituting epidural analgesia for postoperative opioids may also improve outcomes.790
Treating pain after surgery with opioids hinders recurrence, even though opioids promote metastasis.791 A 2018 review of studies concludes “there is no conclusive evidence to avoid the use of opioids with the goal of reducing the risk of recurrence in colorectal cancer.”792
No Increased Risk or Reduced Risk
Non‐steroidal anti‐inflammatory drugs (NSAIDs)
- Use of NSAIDs at the time of surgery was associated with a reduced risk of cancer recurrence after resection for colorectal cancer. No effect was found on five-year mortality or disease-free survival.793 This benefit needs to be balanced with evidence of two increased risks with NSAID use:
- Risk for anastomotic leakage (see sidebar)
- Use of celecoxib (Celebrex) or indomethacin three days before surgery increased tumor infiltration, which could reduce cancer survival following tumor resection.794
- Aspirin use during chemoradiation therapy for rectal cancer before surgery was linked to better progression-free and overall survival.795
- One review found that NSAIDs may decrease tumor growth, with a link to longer recurrence-free survival. Effects for non-selective NSAIDs (aspirin, diclofenac, ibuprofen, naproxen and others) were influenced by the timing and dosage of use.796 Another study found that selective NSAIDs (Celebrex/celecoxib and Mobic/meloxicam) have protective effects on immune function and reduce recurrence and metastasis risk.797
- Ketorolac use before surgery in animals prevented both inflammation and “surgery-induced dormancy escape,” a process that can lead to tumor growth and metastasis.798
- Use of aspirin after surgery is associated with decreased risk of recurrence and death in colorectal cancer.799
Recovery and Remission Maintenance
See Integrative Approaches and Surgery for general information about improving your body terrain to make your body less susceptible to infection, quicker to heal wounds and/or less favorable to cancer.
Survivorship
When you have finished treatment, your cancer treatment team should develop a survivorship plan with you, including these components to help you recover and prevent recurrence:
- Instructions and a schedule for follow-up visits
- Testing
- Guidance on lifestyle and other self-care practices
Post-Treatment Monitoring
The type of testing and monitoring used to assess your response to treatment and detect recurrence will depend on your specific cancer, treatment and risk for recurrence. A valid and reliable test to detect colorectal cancer recurrence early is still needed.
You and your medical team need to find balance with monitoring for colorectal cancer recurrence. Talk with your oncologist about your risk of recurrence and what type and frequency of monitoring is best for you:
- Have we done everything we know to do to treat the cancer?
- What type and frequency of monitoring is best for me?
- What are the monitoring tests and tools available?
The standard monitoring tests are typically are of two types:
- Radiographic scans (such as CAT scans) which involve the risks of significant radiation exposures. The more scans, the higher the risks.
- Measuring CEA (carcinoembryonic antigen) and Ca 19-9 in the blood. Unfortunately, these biomarkers are not good at detecting recurrence.
Neither scans nor tests such as CEA give genetic information about the intrinsic characteristics of each tumor.800
Potential Upcoming Diagnostic Tests
Talk with your doctor about whether one of these new biomarker tests is available for you. Some integrative oncologists are using these new biomarker tests already, but these tests have not been recognized by conventional oncology as a standard in clinical practice.
ctDNA Testing
Blood tests measuring circulating tumor DNA (ctDNA) have generated a lot of excitement and could be a new way to guide treatment decisions or as a trigger to look for residual disease or recurrence. These tests look for biomarkers of cancer recurrence, progression and resistance to therapy. Many potentially useful ctDNA markers are available. A 2019 review found ctDNA tests to be a sensitive and reliable measure of tumor burden.801 The American Society of Clinical Oncology—the main oncology society in the US—considers ctDNA testing promising, but stronger research is needed before it can be recommended for routine use in cancer care.
Measuring Circulating Tumor Cells (CTCs)
Measuring circulating tumor cells (CTCs) in the blood is another test under research and development.802 CTCs are cancer cells that break away from primary or metastatic tumors and enter the bloodstream; they are considered forerunners of metastasis. A 2019 study found that detecting CTCs with a fluid assisted separation technique (FAST) was promising as an early diagnosis tool and biomarker for prognosis in colorectal cancer patients.803 These reviews suggest that CTCs may assist your healthcare team in these tasks:
- Predict survival
- Monitor your response/resistance to treatment
- Assess minimal residual disease
- Find and assess distant metastasis
- Customize therapies in some cases
Even though many difficulties related to CTC testing remain—limiting its use in managing colorectal cancer—reviewers think that their clinical use in colorectal cancer is not far off.804
Commentary
Eggs and Cancer
Integrative naturopathic oncologist and BCCT advisor Lise Alschuler, ND, FABNO, and her colleague Karolyn Gazella advise people with risk for colon cancer to consider limiting egg intake to fewer than five eggs a week, while choosing eggs from free-roaming, organically fed chickens. They also advise boiling or poaching eggs, as these methods do not oxidize the yolk fat.805
- Cancer Treatment Centers of America. Colorectal cancer types. September 15, 2020. Viewed September 16, 2020.
- CancerNet. Colorectal Cancer: Introduction. October 2019. Viewed September 16, 2020.
- American Cancer Society. Colorectal Cancer Signs and Symptoms. June 29, 2020. Viewed September 16, 2020.
- Centers for Disease Control and Prevention. Basic Information About Colorectal Cancer. US Department of Health & Human Services. February 10, 2020. Viewed September 16, 2020.
- American Cancer Society. Colorectal Cancer Facts & Figures 2020-2022. Viewed November 11, 2020.
- Siegel RL, Fedewa SA et al. Colorectal cancer incidence patterns in the United States, 1974-2013. Journal of the National Cancer Institute. 2017 Aug 1;109(8).
- Arhi CS, Ziprin P et al. Colorectal cancer patients under the age of 50 experience delays in primary care leading to emergency diagnoses: a population-based study. Colorectal Disease. 2019 Nov;21(11):1270-1278.
- Baak JP, Gyllenhaal C, Liu L, Guo H, Block KI. Prognostic proof and possible therapeutic mechanisms of herbal medicine in patients with metastatic lung and colon cancer. Integrative Cancer Therapies. 2011 Sep;10(3):NP1-NP11.
- Block KI, Block PB, Gyllenhaal C. Integrative treatment for colorectal cancer: a comprehensive approach. The Journal of Alternative And Complementary Medicine. 2018 Sep/Oct;24(9-10):890-901.
- Steele SR, Chang GJ et al. Practice guideline for the surveillance of patients after curative treatment of colon and rectal cancer. Diseases of the Colon and Rectum. 2015 Aug;58(8):713-25.
- Bibbins-Domingo K; US Preventive Services Task Force. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: US Preventive Services Task Force recommendation statement. Annals of Internal Medicine. 2016 Jun 21;164(12):836-45.
- Leanna Standish. Email communication with Laura Pole. September 28, 2018.
- Integrative oncology study draws attention for promising results. Bastyr University News. December 4, 2013 Viewed May 17, 2019.
- Survival Rates for Colorectal Cancer. American Cancer Society. June 29, 2020. Viewed November 16, 2020.
- Block KI, Block PB, Gyllenhaal C. Integrative treatment for colorectal cancer: a comprehensive approach. Journal of Alternative and Complementary Medicine. 2018 Sep/Oct;24(9-10):890-901.
- Alschuler LN, Gazella KA. The Definitive Guide to Cancer, 3rd Edition: An Integrative Approach to Prevention, Treatment, and Healing. Berkeley, California: Celestial Arts. 2010; Alschuler LN, Gazella KA. The Definitive Guide to Thriving after Cancer: A Five-Step Integrative Plan to Reduce the Risk of Recurrence and Build Lifelong Health. Berkeley, California: Ten Speed Press. 2013.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Cohen L, Jefferies A. Anticancer Living: Transform Your Life and Health with the Mix of Six. New York: Viking. 2018.
- Lemole G, Mehta P, McKee D. After Cancer Care: The Definitive Self-Care Guide to Getting and Staying Well for Patients with Cancer. New York, New York: Rodale, Inc. 2015.
- McKinney N. Naturopathic Oncology, 3rd Edition. Victoria, BC, Canada: Liaison Press. 2016.
- Parmar G, Kaczor T. Textbook of Naturopathic Oncology: A Desktop Guide of Integrative Cancer Care. 1st edition. Canada: Medicatrix Holdings Ltd. 2020.
- Chan KK, Yao TJ et al. The use of Chinese herbal medicine to improve quality of life in women undergoing chemotherapy for ovarian cancer: a double-blind placebo-controlled randomized trial with immunological monitoring. Annals of Oncology. 2011 Oct;22(10):2241-9.
- Bae K, Kim E, Choi JJ, Kim MK, Yoo HS. The effectiveness of anticancer traditional Korean medicine treatment on the survival in patients with lung, breast, gastric, colorectal, hepatic, uterine, or ovarian cancer: a prospective cohort study protocol. Medicine (Baltimore). 2018 Oct;97(41):e12444.
- Lemole G, Mehta P, McKee D. After Cancer Care: The Definitive Self-Care Guide to Getting and Staying Well for Patients with Cancer. New York, New York: Rodale, Inc. 2015.
- Romaguera D, Ward H et al. Pre-diagnostic concordance with the WCRF/AICR guidelines and survival in European colorectal cancer patients: a cohort study. BMC Med. 2015 May 7;13:107.
- Van Blarigan EL, Fuchs CS et al. Association of survival with adherence to the American Cancer Society Nutrition and Physical Activity Guidelines for Cancer Survivors after colon cancer diagnosis: The CALGB 89803/Alliance Trial. JAMA Oncology. 2018 Apr 12.
- Matsell SL, Sánchez-García MA, Halliday V, Williams EA, Corfe BM. Investigating the nutritional advice and support given to colorectal cancer survivors in the UK: is it fit for purpose and does it address their needs? Journal of Human Nutrition and Dietetics. 2020 Sep 20.
- Song M, Wu K et al. Low-carbohydrate diet score and macronutrient intake in relation to survival after colorectal cancer diagnosis. JNCI Cancer Spectrum. 2018 Nov;2(4):pky077.
- Volpato M, Hull MA. Omega-3 polyunsaturated fatty acids as adjuvant therapy of colorectal cancer. Cancer Metastasis Reviews. 2018 Sep;37(2-3):545-555; Song M, Ou FS et al. Marine omega-3 fatty acid intake and survival of stage III colon cancer according to tumor molecular markers in NCCTG Phase III trial N0147 (Alliance). International Journal of Cancer. 2019 Jul 15;145(2):380-389; Song M, Zhang X et al. Marine ω-3 polyunsaturated fatty acid intake and survival after colorectal cancer diagnosis. Gut. 2017 Oct;66(10):1790-1796.
- Morales-Oyarvide V, Yuan C et al. Dietary insulin load and cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803 (Alliance). Journal of the National Cancer Institute. 2019 Feb 1;111(2):170-179; Keum N, Yuan C et al. Dietary glycemic and insulin scores and colorectal cancer survival by tumor molecular biomarkers. International Journal of Cancer. 2017 Jun 15;140(12):2648-2656.
- Lee J, Jeon JY, Meyerhardt JA. Diet and lifestyle in survivors of colorectal cancer. Hematology/Oncology Clinics of North America. 2015 Feb;29(1):1-27; Meyerhardt JA, Niedzwiecki D et al. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. JAMA. 2007 Aug 15;298(7):754-64.
- Lee J, Jeon JY, Meyerhardt JA. Diet and lifestyle in survivors of colorectal cancer. Hematology/Oncology Clinics of North America. 2015 Feb;29(1):1-27.
- Abrams DI, Weil AT. Chapter 7, Botanical and Mycological Medicine in Integrative Oncology Integrative Oncology, 2nd Edition. New York, NY: Oxford University Press. 2014.
- Fadelu T, Zhang S et al. Nut consumption and survival in patients with stage iii colon cancer: results from CALGB 89803 (Alliance). Journal of Clinical Oncology. 2018 Feb 28:JCO2017755413.
- Zheng J, Tabung FK et al. Post-cancer diagnosis dietary inflammatory potential is associated with survival among women diagnosed with colorectal cancer in the Women's Health Initiative. European Journal of Nutrition. 2019 Apr 6.
- Wesselink E, Winkels RM et al. Dietary intake of magnesium or calcium and chemotherapy-induced peripheral neuropathy in colorectal cancer patients. Nutrients. 2018 Mar 23;10(4). pii: E398.
- Cleveland Clinic. Magnesium Rich Food. December 1, 2014. Viewed September 16, 2020.
- CancerNet. Peripheral Neuropathy. American Society of Clinical Oncology. May 2017. Viewed March 2, 2020.
- McCulloch M. 15 Healthy Foods High in B Vitamins. Healthline. October 11, 2018. Viewed September 21, 2020.
- Zhu Y, Wu H et al. Dietary patterns and colorectal cancer recurrence and survival: a cohort study. BMJ Open. 2013 Feb 7;3(2). pii: e002270; Meyerhardt JA, Niedzwiecki D et al. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. Journal of the American Medical Association. 2007 Aug 15;298(7):754-64.
- Zhang FF, Cudhea F et al. Preventable cancer burden associated with poor diet in the United States. JNCI Cancer Spectrum. 2019 May;pkz034.
- American Institute for Cancer Research. Colorectal Cancer: Arm yourself with information. December 18, 2019. Viewed September 18, 2020.
- Steele SR, Chang GJ et al. Practice guideline for the surveillance of patients after curative treatment of colon and rectal cancer. Diseases of the Colon and Rectum. 2015 Aug;58(8):713-25.
- Farinetti A, Zurlo V, Manenti A, Coppi F, Mattioli AV. Mediterranean diet and colorectal cancer: a systematic review. Nutrition. 2017 Nov - Dec;43-44:83-88.
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Diet, nutrition, physical activity and colorectal cancer. Viewed May 14, 2019; Thanikachalam K, Khan G. Colorectal cancer and nutrition. Nutrients. 2019 Jan 14;11(1). pii: E164; Meyerhardt JA, Niedzwiecki D et al. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. JAMA. 2007 Aug 15;298(7):754-64; Song M, Chan AT. Environmental factors, gut microbiota, and colorectal cancer prevention. Clinical Gastroenterology and Hepatology. 2019 Jan;17(2):275-289; Murphy N, Norat T et al. Consumption of dairy products and colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC). PLoS One. 2013 Sep 2;8(9):e72715; Larsson SC, Bergkvist L, Wolk A. High-fat dairy food and conjugated linoleic acid intakes in relation to colorectal cancer incidence in the Swedish Mammography Cohort. American Journal of Clinical Nutrition. 2005 Oct;82(4):894-900; Bailie L, Loughrey MB, Coleman HG. Lifestyle risk factors for serrated colorectal polyps: a systematic review and meta-analysis. Gastroenterology. 2017 Jan;152(1):92-104; Kronborg O.Endoscopy. Colon polyps and cancer. 2004 Jan;36(1):3-7; Katona BW, Weiss JM. Chemoprevention of colorectal cancer. Gastroenterology. 2020;158(2):368–388; Chapelle N, Martel M, Toes-Zoutendijk E, Barkun AN, Bardou M. Recent advances in clinical practice: colorectal cancer chemoprevention in the average-risk population. Gut. 2020 Sep 28:gutjnl-2020-320990; Veettil SK, Wong TY et al. Role of diet in colorectal cancer incidence: umbrella review of meta-analyses of prospective observational studies. JAMA Network Open. 2021 Feb 1;4(2):e2037341.
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Diet, nutrition, physical activity and colorectal cancer. Viewed May 14, 2019; Bradbury KE, Murphy N, Key TJ. Diet and colorectal cancer in UK Biobank: a prospective study. International Journal of Epidemiology. 2019 Apr 17. pii: dyz064; Meyerhardt JA, Niedzwiecki D et al. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. JAMA. 2007 Aug 15;298(7):754-64; Bailie L, Loughrey MB, Coleman HG. Lifestyle risk factors for serrated colorectal polyps: a systematic review and meta-analysis. Gastroenterology. 2017 Jan;152(1):92-104; Dolejs SC, Gayed B, Fajardo A. Prevention of colorectal neoplasia. Clinics in Colon and Rectal Surgery. 2016;29(4):353-362; Chapelle N, Martel M, Toes-Zoutendijk E, Barkun AN, Bardou M. Recent advances in clinical practice: colorectal cancer chemoprevention in the average-risk population. Gut. 2020 Sep 28:gutjnl-2020-320990; Veettil SK, Wong TY et al. Role of diet in colorectal cancer incidence: umbrella review of meta-analyses of prospective observational studies. JAMA Network Open. 2021 Feb 1;4(2):e2037341.
- Thanikachalam K, Khan G. Colorectal cancer and nutrition. Nutrients. 2019 Jan 14;11(1). pii: E164; Bultman SJ. Emerging roles of the microbiome in cancer. Carcinogenesis. 2014;35(2):249–255.
- Katona BW, Weiss JM. Chemoprevention of colorectal cancer. Gastroenterology. 2020;158(2):368–388.
- Beresford SA, Johnson KC et al. Low-fat dietary pattern and risk of colorectal cancer: the Women's Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006 Feb 8;295(6):643-54.
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Diet, nutrition, physical activity and colorectal cancer. Viewed May 14, 2019; Durko L, Malecka-Panas E et al. Lifestyle modifications and colorectal cancer. Current Colorectal Cancer Reports. 2014;10:45-54; Thanikachalam K, Khan G. Colorectal cancer and nutrition. Nutrients. 2019 Jan 14;11(1). pii: E164; Durko L, Malecka-Panas E et al. Lifestyle modifications and colorectal cancer. Current Colorectal Cancer Reports. 2014;10:45-54; Thanikachalam K, Khan G. Colorectal cancer and nutrition. Nutrients. 2019 Jan 14;11(1). pii: E164; Shivappa N, Godos J et al. Dietary inflammatory index and colorectal cancer risk-a meta-analysis. Nutrients. 2017 Sep 20;9(9). pii: E1043; Tabung FK, Liu L et al. Association of dietary inflammatory potential with colorectal cancer risk in men and women. JAMA Oncology. 2018 Mar 1;4(3):366-373; Bailie L, Loughrey MB, Coleman HG. Lifestyle risk factors for serrated colorectal polyps: a systematic review and meta-analysis. Gastroenterology. 2017 Jan;152(1):92-104; Green CJ, de Dauwe P et al. Tea, coffee, and milk consumption and colorectal cancer risk. Journal of Epidemiology. 2014;24(2):146-53; Su LJ, Arab L. Tea consumption and the reduced risk of colon cancer—results from a national prospective cohort study. Public Health Nutrition. 2002 Jun;5(3):419-25; Wada K, Oba S et al. Green tea intake and colorectal cancer risk in Japan: the Takayama study. Japanese Journal of Clinical Oncology. 2019 Jun 1;49(6):515-520; Ullah MF, Bhat SH et al. Pharmacological intervention through dietary nutraceuticals in gastrointestinal neoplasia. Critical Reviews in Food Science and Nutrition. 2016 Jul 3;56(9):1501-18; Arab L, Il'yasova D. The epidemiology of tea consumption and colorectal cancer incidence. Journal of Nutrition. 2003 Oct;133(10):3310S-3318S; Chen Y, Wu Y et al. An inverse association between tea consumption and colorectal cancer risk. Oncotarget. 2017 Jun 6;8(23):37367-37376; Sun CL, Yuan JM, Koh WP, Yu MC. Green tea, black tea and colorectal cancer risk: a meta-analysis of epidemiologic studies. Carcinogenesis. 2006 Jul;27(7):1301-9; Mackintosh C, Yuan C et al. Association of coffee intake with survival in patients with advanced or metastatic colorectal cancer. JAMA Oncology. 2020;10.1001/jamaoncol.2020.3938; Ngo SN, Williams DB, Cobiac L, Head RJ. Does garlic reduce risk of colorectal cancer? A systematic review. Journal of Nutrition. 2007 Oct;137(10):2264-9; Fleischauer AT, Poole C, Arab L. Garlic consumption and cancer prevention: meta-analyses of colorectal and stomach cancers. American Journal of Clinical Nutrition. 2000 Oct;72(4):1047-52; Bobe G, Sansbury LB et al. Dietary flavonoids and colorectal adenoma recurrence in the Polyp Prevention Trial. Cancer Epidemiology, Biomarkers & Prevention. 2008;17(6):1344-1353; Chang H, Lei L, Zhou Y, Ye F, Zhao G. Dietary flavonoids and the risk of colorectal cancer: an updated meta-analysis of epidemiological studies. Nutrients. 2018;10(7):950; Zheng X, Wu K et al. Yogurt consumption and risk of conventional and serrated precursors of colorectal cancer. Gut. 2019;gutjnl-2019-318374; Chapelle N, Martel M, Toes-Zoutendijk E, Barkun AN, Bardou M. Recent advances in clinical practice: colorectal cancer chemoprevention in the average-risk population. Gut. 2020 Sep 28:gutjnl-2020-320990; Touvier M, Chan DS et alT. Meta-analyses of vitamin D intake, 25-hydroxyvitamin D status, vitamin D receptor polymorphisms, and colorectal cancer risk. Cancer Epidemiology, Biomarkers & Prevention. 2011 May;20(5):1003-16; ; Veettil SK, Wong TY et al. Role of diet in colorectal cancer incidence: umbrella review of meta-analyses of prospective observational studies. JAMA Network Open. 2021 Feb 1;4(2):e2037341; Kim DH, Smith-Warner SA et al. Pooled analyses of 13 prospective cohort studies on folate intake and colon cancer. Cancer Causes & Control. 2010 Nov;21(11):1919-30; Gibson TM, Weinstein SJ et al. Pre- and postfortification intake of folate and risk of colorectal cancer in a large prospective cohort study in the United States. American Journal of Clinical Nutrition. 2011 Oct;94(4):1053-62; Giovannucci E. Epidemiologic studies of folate and colorectal neoplasia: a review. Journal of Nutrition. 2002 Aug;132(8 Suppl):2350S-2355S.
- Fleischauer AT, Arab L. Garlic and cancer: a critical review of the epidemiologic literature. Journal of Nutrition. 2001 Mar;131(3s):1032S-40S; Fleischauer AT, Poole C, Arab L. Garlic consumption and cancer prevention: meta-analyses of colorectal and stomach cancers. American Journal of Clinical Nutrition. 2000 Oct;72(4):1047-52.
- Chiavarini M, Minelli L, Fabiani R. Garlic consumption and colorectal cancer risk in man: a systematic review and meta-analysis. Public Health Nutrition. 2016 Feb;19(2):308-17; Baena R, Salinas P. Diet and colorectal cancer. Maturitas. 2015 Mar;80(3):258-64; Hu JY, Hu YW et al. Consumption of garlic and risk of colorectal cancer: an updated meta-analysis of prospective studies. World Journal of Gastroenterology. 2014 Nov 7;20(41):15413-22; Chapelle N, Martel M, Toes-Zoutendijk E, Barkun AN, Bardou M. Recent advances in clinical practice: colorectal cancer chemoprevention in the average-risk population. Gut. 2020 Sep 28:gutjnl-2020-320990; Wan Q, Li N et al. Allium vegetable consumption and health: an umbrella review of meta-analyses of multiple health outcomes. Food Science & Nutrition. 2019 Jul 10;7(8):2451-2470.
- Chapelle N, Martel M, Toes-Zoutendijk E, Barkun AN, Bardou M. Recent advances in clinical practice: colorectal cancer chemoprevention in the average-risk population. Gut. 2020 Sep 28:gutjnl-2020-320990.
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Diet, nutrition, physical activity and colorectal cancer. Viewed October 19, 2020.
- Thanikachalam K, Khan G. Colorectal cancer and nutrition. Nutrients. 2019 Jan 14;11(1). pii: E164; Foods that fight inflammation. Harvard Health Publishing. August 29, 2020. Viewed October 19, 2020.
- Dolejs SC, Gayed B, Fajardo A. Prevention of colorectal neoplasia. Clinics in Colon and Rectal Surgery. 2016;29(4):353-362.
- Zhang SM, Moore SC et al. Folate, vitamin B6, multivitamin supplements, and colorectal cancer risk in women. American Journal of Epidemiology. 2006;163(2):108–115; de Vogel S, Dindore V et al. Dietary folate, methionine, riboflavin, and vitamin B-6 and risk of sporadic colorectal cancer. Journal of Nutrition. 2008;138(12):2372–2378; Ben S, Du M et al. Vitamin B2 intake reduces the risk for colorectal cancer: a dose-response analysis. European Journal of Nutrition. 2019;58(4):1591–1602.
- Schernhammer ES, Giovannuccci E, Fuchs CS, Ogino S. A prospective study of dietary folate and vitamin B and colon cancer according to microsatellite instability and KRAS mutational status. Cancer Epidemiology, Biomarkers & Prevention. 2008;17(10):2895–2898.
- Banjari I, Kožić S. Dietary intake of vitamin B12 in relation to diet and lifestyle characteristics in a population at high risk for colorectal cancer. Central European Journal of Public Health. 2018;26(4):253–259.
- National Health Service. B vitamins and folic acid. August 20, 2020. Viewed September 16, 2020.
- Zhang SM, Moore SC et al. Folate, vitamin B6, multivitamin supplements, and colorectal cancer risk in women. American Journal of Epidemiology. 2006;163(2):108–115; Schernhammer ES, Giovannuccci E, Fuchs CS, Ogino S. A prospective study of dietary folate and vitamin B and colon cancer according to microsatellite instability and KRAS mutational status. Cancer Epidemiology, Biomarkers & Prevention. 2008;17(10):2895–2898.
- Weinstein SJ, Albanes D, Selhub J, et al. One-carbon metabolism biomarkers and risk of colon and rectal cancers. Cancer Epidemiology, Biomarkers and Prevention. 2008;17(11):3233–3240.
- Jia K, Wang R, Tian J. Vitamin B6 intake and the risk of colorectal cancer: a meta-analysis of prospective cohort studies. Nutrition and Cancer. 2017;69(5):723–731.
- de Vogel S, Dindore V et al. Dietary folate, methionine, riboflavin, and vitamin B-6 and risk of sporadic colorectal cancer. Journal of Nutrition. 2008;138(12):2372–2378.
- Zhang SM, Moore SC et al. Folate, vitamin B6, multivitamin supplements, and colorectal cancer risk in women. American Journal of Epidemiology. 2006;163(2):108–115.
- Yang CY, Chiu HF, Chiu JF, Tsai SS, Cheng MF. Calcium and magnesium in drinking water and risk of death from colon cancer. Japanese Journal of Cancer Research. 1997 Oct;88(10):928-33.
- Chen GC, Pang Z, Liu QF. Magnesium intake and risk of colorectal cancer: a meta-analysis of prospective studies. European Journal of Clinical Nutrition. 2012 Nov;66(11):1182-6; Crosara Teixeira M, Braghiroli MI, Sabbaga J, Hoff PM. Primary prevention of colorectal cancer: myth or reality? World Journal of Gastroenterology. 2014 Nov 7;20(41):15060-9; Thanikachalam K, Khan G. Colorectal cancer and nutrition. Nutrients. 2019 Jan 14;11(1). pii: E164.
- Zhu X, Shrubsole MJ et al. Calcium/magnesium intake ratio, but not magnesium intake, interacts with genetic polymorphism in relation to colorectal neoplasia in a two-phase study. Molecular Carcinogenesis. 2016 Oct;55(10):1449-57; Bailie L, Loughrey MB, Coleman HG. Lifestyle risk factors for serrated colorectal polyps: a systematic review and meta-analysis. Gastroenterology. 2017 Jan;152(1):92-104; Song M, Chan AT. Environmental factors, gut microbiota, and colorectal cancer prevention. Clinical Gastroenterology and Hepatology. 2019 Jan;17(2):275-289.
- Orchel A, Dzierzewicz Z, Parfiniewicz B, Weglarz L, Wilczok T. Butyrate-induced differentiation of colon cancer cells is PKC and JNK dependent. Dig Dis Sci. 2005 Mar;50(3):490-8.
- Bultman SJ. Emerging roles of the microbiome in cancer. Carcinogenesis. 2014;35(2):249–255.
- Liu K, Zhou R et al. Effect of green tea on glucose control and insulin sensitivity: a meta-analysis of 17 randomized controlled trials. American Journal of Clinical Nutrition. 2013 Aug;98(2):340-8.
- Martín MA, Goya L, Ramos S. Preventive effects of cocoa and cocoa antioxidants in colon cancer. Diseases. 2016;4(1):6.
- Qu B, Qu H. The influence of statins on risk and patient survival in colorectal cancer. Journal of Clinical Gastroenterology. 2019;53(9):699–701.
- Meyerhardt JA, Giovannucci EL et al. Physical activity and survival after colorectal cancer diagnosis. Journal of Clinical Oncology. 2006 Aug 1;24(22):3527-34; Lee J, Jeon JY, Meyerhardt JA. Diet and lifestyle in survivors of colorectal cancer. Hematology/Oncology Clinics of North America. 2015 Feb;29(1):1-27; Li T, Wei S et al. The dose-response effect of physical activity on cancer mortality: findings from 71 prospective cohort studies. British Journal of Sports Medicine. 2016 Mar;50(6):339-45; Ratjen I, Schafmayer C et al. Postdiagnostic physical activity, sleep duration, and TV watching and all-cause mortality among long-term colorectal cancer survivors: a prospective cohort study. BMC Cancer. 2017 Oct 25;17(1):701.
- Marshall CH, Al-Mallah MH et al. Cardiorespiratory fitness and incident lung and colorectal cancer in men and women: results from the Henry Ford Exercise Testing (FIT) cohort. Cancer. 2019 May 6.
- Van Blarigan EL, Meyerhardt JA. Role of physical activity and diet after colorectal cancer diagnosis. Journal of Clinical Oncology. 2015 Jun 1;33(16):1825-34.
- Harvard Women's Health Watch. MET-hour equivalents of various physical activities. Harvard Health Publishing. December 2009. Viewed February 27, 2019.
- Blanchard CM, Courneya KS, Stein K; American Cancer Society's SCS-II. Cancer survivors' adherence to lifestyle behavior recommendations and associations with health-related quality of life: results from the American Cancer Society's SCS-II. Journal of Clinical Oncology. 2008 May 1;26(13):2198-204.
- Johnson BL, Trentham-Dietz A, Koltyn KF, Colbert LH. Physical activity and function in older, long-term colorectal cancer survivors. Cancer Causes & Control. 2009 Jul;20(5):775-84.
- Lynch BM, Cerin E, Owen N, Aitken JF. Associations of leisure-time physical activity with quality of life in a large, population-based sample of colorectal cancer survivors. Cancer Causes & Control. 2007 Sep;18(7):735-42; Courneya KS, Friedenreich CM. Physical exercise and quality of life following cancer diagnosis: a literature review. Annals of Behavioral Medicine. 1999 Spring;21(2):171-9.
- Peddle CJ, Au HJ, Courneya KS. Associations between exercise, quality of life, and fatigue in colorectal cancer survivors. Diseases of the Colon and Rectum. 2008 Aug;51(8):1242-8.
- Courneya KS, Friedenreich CM et al. A randomized trial of exercise and quality of life in colorectal cancer survivors. European Journal of Cancer Care (Engl). 2003 Dec;12(4):347-57.
- Courneya KS, Friedenreich CM. Relationship between exercise pattern across the cancer experience and current quality of life in colorectal cancer survivors. Journal of Alternative and Complementary Medicine. 1997 Fall;3(3):215-26.
- Peddle CJ, Au HJ, Courneya KS. Associations between exercise, quality of life, and fatigue in colorectal cancer survivors. Diseases of the Colon and Rectum. 2008 Aug;51(8):1242-8.
- Courneya KS, Friedenreich CM. Physical exercise and quality of life following cancer diagnosis: a literature review. Annals of Behavioral Medicine. 1999 Spring;21(2):171-9.
- Courneya KS, Friedenreich CM. Physical exercise and quality of life following cancer diagnosis: a literature review. Annals of Behavioral Medicine. 1999 Spring;21(2):171-9.
- Exercising for Better Sleep. Johns Hopkins Medicine. Viewed November 25, 2020.
- Coles T, Bennett AV et al. Relationship between sleep and exercise as colorectal cancer survivors transition off treatment. Supportive Care in Cancer. 2018 Aug;26(8):2663-2673.
- Steele SR, Chang GJ et al. Practice guideline for the surveillance of patients after curative treatment of colon and rectal cancer. Diseases of the Colon and Rectum. 2015 Aug;58(8):713-25.
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Diet, nutrition, physical activity and colorectal cancer. Viewed May 14, 2019; Samad AK, Taylor RS, Marshall T, Chapman MA. A meta-analysis of the association of physical activity with reduced risk of colorectal cancer. Colorectal Disease. 2005 May;7(3):204-13; Wolin KY, Yan Y, Colditz GA, Lee IM. Physical activity and colon cancer prevention: a meta-analysis. British Journal of Cancer. 2009 Feb 24;100(4):611-6; Dolejs SC, Gayed B, Fajardo A. Prevention of colorectal neoplasia. Clinics in Colon and Rectal Surgery. 2016;29(4):353-362.
- Marshall CH, Al-Mallah MH et al. Cardiorespiratory fitness and incident lung and colorectal cancer in men and women: results from the Henry Ford Exercise Testing (FIT) cohort. Cancer. 2019 May 6; Crosara Teixeira M, Braghiroli MI, Sabbaga J, Hoff PM. Primary prevention of colorectal cancer: myth or reality? World Journal of Gastroenterology. 2014 Nov 7;20(41):15060-9; Song M, Chan AT. Environmental factors, gut microbiota, and colorectal cancer prevention. Clinical Gastroenterology and Hepatology. 2019 Jan;17(2):275-289.
- Matthews CE, Moore SC et al. Amount and intensity of leisure-time physical activity and lower cancer risk. Journal of Clinical Oncology. 2019 Dec 26:JCO1902407.
- Kikuchi N, Nishiyama T et al. Perceived stress and colorectal cancer incidence: the Japan Collaborative Cohort Study. Scientific Reports. 2017 Jan 16;7:40363.
- Xiao Q, Arem H, Pfeiffer R, Matthews C. Prediagnosis sleep duration, napping, and mortality among colorectal cancer survivors in a large US cohort. Sleep. 2017 Apr 1;40(4).
- Xiao Q, Arem H, Pfeiffer R, Matthews C. Prediagnosis sleep duration, napping, and mortality among colorectal cancer survivors in a large US cohort. Sleep. 2017 Apr 1;40(4).
- Ratjen I, Schafmayer C et al. Postdiagnostic physical activity, sleep duration, and TV watching and all-cause mortality among long-term colorectal cancer survivors: a prospective cohort study. BMC Cancer. 2017 Oct 25;17(1):701.
- Mormont MC, Waterhouse J et al. Marked 24-h rest/activity rhythms are associated with better quality of life, better response, and longer survival in patients with metastatic colorectal cancer and good performance status. Clinical Cancer Research. 2000 Aug;6(8):3038-45.
- Innominato PF, Giacchetti S et al. Prediction of overall survival through circadian rest-activity monitoring during chemotherapy for metastatic colorectal cancer. International Journal of Cancer. 2012 Dec 1;131(11):2684-92.
- Innominato PF, Giacchetti S et al. Fatigue and weight loss predict survival on circadian chemotherapy for metastatic colorectal cancer. Cancer. 2013 Jul 15;119(14):2564-73.
- Mormont MC, Waterhouse J et al. Marked 24-h rest/activity rhythms are associated with better quality of life, better response, and longer survival in patients with metastatic colorectal cancer and good performance status. Clinical Cancer Research. 2000 Aug;6(8):3038-45; Stouffer J, Block PB et al. Correlations between circadian rhythms and quality of life in cancer patients. Presented at the annual meeting of the Society for Integrative Oncology, November 2006, Boston Massachusetts.
- Coles T, Bennett AV et al. Relationship between sleep and exercise as colorectal cancer survivors transition off treatment. Supportive Care Cancer. 2018 Aug;26(8):2663-2673.
- Steele SR, Chang GJ et al. Practice guideline for the surveillance of patients after curative treatment of colon and rectal cancer. Diseases of the Colon and Rectum. 2015 Aug;58(8):713-25.
- Durko L, Malecka-Panas E et al. Lifestyle modifications and colorectal cancer. Current Colorectal Cancer Reports. 2014;10:45-54.
- Zhao H, Yin JY et al. Sleep duration and cancer risk: a systematic review and meta-analysis of prospective studies. Asian Pacific Journal of Cancer Prevention. 2013;14(12):7509-15.
- Dun A, Zhao X et al. Association between night-shift work and cancer risk: updated systematic review and meta-analysis. Frontiers in Oncology. 2020 Jun 23;10:1006.
- Block KI, Block PB, Gyllenhaal C. Integrative treatment for colorectal cancer: a comprehensive approach. Journal of Alternative and Complementary Medicine. 2018 Sep/Oct;24(9-10):890-901.
- Janssen S, Solomon G, SchettlerT. Toxicant and Disease Database. Collaborative on Health and the Environment. 2011. Viewed May 20, 2019; Ward MH, Jones RR. Drinking water nitrate and human health: an updated review. International Journal of Environmental Research and Public Health. 2018 Jul 23;15(7). pii: E1557.
- Nogacka AM, Gómez-Martín M et al. Xenobiotics formed during food processing: their relation with the intestinal microbiota and colorectal cancer. International Journal of Molecular Sciences. 2019 Apr 25;20(8). pii: E2051.
- Kotronoulas G, Papadopoulou C, Burns-Cunningham K, Simpson M, Maguire R. A systematic review of the supportive care needs of people living with and beyond cancer of the colon and/or rectum. European Journal of Oncology Nursing. 2017 Aug;29:60-70.
- Goldzweig G, Hubert A et al. Gender and psychological distress among middle- and older-aged colorectal cancer patients and their spouses: an unexpected outcome. Critical Reviews in Oncology/Hematology. 2009 Apr;70(1):71-82.
- Gonzalez-Saenz de Tejada M, Bilbao A et al. Association between social support, functional status, and change in health-related quality of life and changes in anxiety and depression in colorectal cancer patients. Psychooncology. 2017 Sep;26(9):1263-1269.
- Dong X, Li G2 et al. The mediating role of resilience in the relationship between social support and posttraumatic growth among colorectal cancer survivors with permanent intestinal ostomies: A structural equation model analysis. European Journal of Oncology Nursing. 2017 Aug;29:47-52.
- Haviland J, Sodergren S et al. Social support following diagnosis and treatment for colorectal cancer and associations with health-related quality of life: results from the UK ColoREctal Wellbeing (CREW) cohort study. Psychooncology. 2017 Dec;26(12):2276-2284.
- Creagan ET. Psychosocial issues in oncologic practice. Mayo Clinic Proceedings. 1993 Feb;68(2):161-7.
- Rogers CR, Mitchell JA, Franta GJ, Foster MJ, Shires D. Masculinity, racism, social support, and colorectal cancer screening uptake among African American men: a systematic review. American Journal of Men’s Health. 2017 Sep;11(5):1486-1500.
- Dunn J, Lynch B et al. Dimensions of quality of life and psychosocial variables most salient to colorectal cancer patients. Psychooncology. 2006 Jan;15(1):20-30.
- Davenport L. Drug-drug interactions to avoid in patients with GI cancer. Medscape Oncology. July 8, 2020. Viewed July 13, 2020.
- Alyami M, Hübner M et al. Pressurised intraperitoneal aerosol chemotherapy: rationale, evidence, and potential indications. Lancet Oncology. 2019 Jul;20(7):e368-e377.
- Pulsed low-dose pelvic reirradiation safe, provided tangible benefit for patients with few other options. Fox Chase Cancer Center, September 22, 2020. Viewed September 30, 2020; Paly JJ, Deng M et al. Pelvic reirradiation utilizing pulsed low-dose rate radiation therapy. American Journal of Clinical Oncology. 2020 Oct;43(10):748-751.
- Baak JP, Gyllenhaal C, Liu L, Guo H, Block KI. Prognostic proof and possible therapeutic mechanisms of herbal medicine in patients with metastatic lung and colon cancer. Integrative Cancer Therapies. 2011 Sep;10(3):NP1-NP11.
- Smith JJ, Strombom P et al. Assessment of a watch-and-wait strategy for rectal cancer in patients with a complete response after neoadjuvant therapy. JAMA Oncology. 2019 Jan 10:e185896.
- Hanna TP, King WD et al. Mortality due to cancer treatment delay: systematic review and meta-analysis. BMJ. 2020 Nov 4;371:m4087; Reinberg S. Delaying cancer care costs lives. HealthDay. November 5, 2020. Viewed November 8, 2020.
- Inpatient Surgery Report 2019. The Leapfrog Group. Viewed September 4, 2019.
- Leeds IL, Meyers PM et al. Psychosocial risks are independently associated with cancer surgery outcomes in medically comorbid patients. Annals of Surgical Oncology. 2019 Apr;26(4):936-944.
- Harvie M. Nutritional supplements and cancer: potential benefits and proven harms. American Society of Clinical Oncology Educational Book. 2014;e478-e486.
- Harvie M. Nutritional supplements and cancer: potential benefits and proven harms. American Society of Clinical Oncology Educational Book. 2014;e478-e486.
- Harvie M. Nutritional supplements and cancer: potential benefits and proven harms. American Society of Clinical Oncology Educational Book. 2014;e478-e486.
- Papaioannou D, Cooper KL et al. Antioxidants in the chemoprevention of colorectal cancer and colorectal adenomas in the general population: a systematic review and meta-analysis. Colorectal Disease. 2011;13(10):1085–1099; Song M, Garrett WS, Chan AT. Nutrients, foods, and colorectal cancer prevention. Gastroenterology. 2015;148(6):1244-60.e16; Katona BW, Weiss JM. Chemoprevention of colorectal cancer. Gastroenterology. 2020;158(2):368-388.
- Bonelli L, Puntoni M et al. Antioxidant supplement and long-term reduction of recurrent adenomas of the large bowel. A double-blind randomized trial. Journal of Gastroenterology. 2013;48(6):698-705.
- Harvie M. Nutritional supplements and cancer: potential benefits and proven harms. American Society of Clinical Oncology Educational Book. 2014;e478-e486.
- Sakamoto J, Morita S et al. Efficacy of adjuvant immunochemotherapy with polysaccharide K for patients with curatively resected colorectal cancer: a meta-analysis of centrally randomized controlled clinical trials. Cancer Immunology, Immunotherapy: CII. 2006 Apr;55(4):404-11; Ramberg JE, Nelson ED, Sinnott RA. Immunomodulatory dietary polysaccharides: a systematic review of the literature. Nutrition Journal. 2010 Nov 18;9:54; Torisu M, Hayashi Y et al. Significant prolongation of disease-free period gained by oral polysaccharide K (PSK) administration after curative surgical operation of colorectal cancer. Cancer Immunology, Immunotherapy. 1990;31(5):261–268; Mitomi T, Tsuchiya S et al. Randomized, controlled study on adjuvant immunochemotherapy with PSK in curatively resected colorectal cancer. the Cooperative Study Group of Surgical Adjuvant Immunochemotherapy for Cancer of Colon and Rectum (Kanagawa). Diseases of the Colon and Rectum. 1992;35(2):123–130.
- Evidence-Based Monographs: Professional Resource: Coriolus Versicolor. Ottawa Integrative Cancer Centre. Viewed June 6, 2019.
- Ohwada S, Ikeya T et al. Adjuvant immunochemotherapy with oral Tegafur/Uracil plus PSK in patients with stage II or III colorectal cancer: a randomised controlled study. British Journal of Cancer. 2004 Mar 8;90(5):1003-10.
- Yamashita K, Ougolkov AV et al. Adjuvant immunochemotherapy with protein-bound polysaccharide K for colon cancer in relation to oncogenic beta-catenin activation. Diseases of the Colon and Rectum. 2007 Aug;50(8):1169-81.
- Sakai T, Yamashita Y, Maekawa T, Mikami K, Hoshino S, Shirakusa T. Immunochemotherapy with PSK and fluoropyrimidines improves long-term prognosis for curatively resected colorectal cancer. Cancer Biotherapy & Radiopharmaceuticals. 2008 Aug;23(4):461-7.
- Abrams DI, Weil AT. Integrative Oncology, 2nd Edition. New York, NY: Oxford University Press. 2014.
- Borchers AT, Stern JS, Hackman RM, Keen CL, Gershwin ME. Mushrooms, tumors, and immunity. Proceedings of the Society for Experimental Biology and Medicine. 1999;221(4):281–293.
- Li YH, Niu YB et al. Role of phytochemicals in colorectal cancer prevention. World Journal of Gastroenterology. 2015 Aug 21;21(31):9262-72.
- Alschuler LN, Gazella KA. The Definitive Guide to Cancer, 3rd Edition: An Integrative Approach to Prevention, Treatment, and Healing. Berkeley, California: Celestial Arts. 2010; Alschuler LN, Gazella KA. The Definitive Guide to Thriving after Cancer: A Five-Step Integrative Plan to Reduce the Risk of Recurrence and Build Lifelong Health. Berkeley, California: Ten Speed Press. 2013.
- McKinney N. Naturopathic Oncology, 3rd Edition. Victoria, BC, Canada: Liaison Press. 2016.
- Maalmi H, Ordóñez-Mena JM, Schöttker B, Brenner H. Serum 25-hydroxyvitamin D levels and survival in colorectal and breast cancer patients: systematic review and meta-analysis of prospective cohort studies. European Journal of Cancer. 2014 May;50(8):1510-21; Shang M, Sun J. Vitamin D/VDR, probiotics, and gastrointestinal diseases. Current Medicinal Chemistry. 2017;24(9):876-887.
- Urashima M, Ohdaira H et al. Effect of vitamin D supplementation on relapse-free survival among patients with digestive tract cancers: the AMATERASU randomized clinical trial. JAMA. 2019 Apr 9;321(14):1361-1369.
- Ng K, Nimeiri HS et al. Effect of high-dose vs standard-dose vitamin D3 supplementation on progression-free survival among patients with advanced or metastatic colorectal cancer: the SUNSHINE randomized clinical trial. JAMA. 2019 Apr 9;321(14):1370-1379.
- Lin S, An X, Guo Y, et al. Meta-analysis of astragalus-containing traditional Chinese medicine combined with chemotherapy for colorectal cancer: efficacy and safety to tumor response. Frontiers in Oncology. 2019;9:749; Abrams DI, Weil AT. Integrative Oncology, 2nd Edition. New York, NY: Oxford University Press. 2014; Chen M, May BH, Zhou IW, Xue CC, Zhang AL. FOLFOX 4 combined with herbal medicine for advanced colorectal cancer: a systematic review. Phytotherapy Research. 2014;28(7):976–991.
- Ong SKL, Shanmugam MK et al. Focus on formononetin: anticancer potential and molecular targets. Cancers (Basel). 2019;11(5):611; Dong J, Liang W et al. Saponins regulate intestinal inflammation in colon cancer and IBD. Pharmacological Research. 2019;144:66–72.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS Journal. 2013 Jan;15(1):195-218.
- Greil R, Greil-Ressler S et al. A phase 1 dose-escalation study on the safety, tolerability and activity of liposomal curcumin (Lipocurc™) in patients with locally advanced or metastatic cancer. Cancer Chemotherapy and Pharmacology. 2018 Oct;82(4):695-706.
- Sahebkar A. Are curcuminoids effective C-reactive protein-lowering agents in clinical practice? Evidence from a meta-analysis. Phytotherapy Research. 2014 May;28(5):633-42.
- Patel BB, Majumdar AP. Synergistic role of curcumin with current therapeutics in colorectal cancer: minireview. Nutrition and Cancer. 2009;61(6):842-6; Jalili-Nik M, Soltani A et al. Current status and future prospective of curcumin as a potential therapeutic agent in the treatment of colorectal cancer. Journal of Cellular Physiology. 2018 Sep;233(9):6337-6345.
- Howells LM, Iwuji COO et al. Curcumin combined with FOLFOX chemotherapy is safe and tolerable in patients with metastatic colorectal cancer in a randomized phase IIa trial. Journal of Nutrition. 2019 Jul 1;149(7):1133-1139.
- Ismail NI, Othman I, Abas F, H Lajis N, Naidu R.l. Mechanism of apoptosis induced by curcumin in colorectal cancer. International Journal of Molecular Sciiences. 2019 May 17;20(10). pii: E2454.
- Ravindran J, Prasad S, Aggarwal BB. Curcumin and cancer cells: How many ways can curry kill tumor cells selectively? AAPS J. 2009 Sep;11(3):495-510.
- Huang YF, Zhu DJ et al. Curcumin enhances the effects of irinotecan on colorectal cancer cells through the generation of reactive oxygen species and activation of the endoplasmic reticulum stress pathway. Oncotarget. 2017 Jun 20;8(25):40264-40275.
- Noratto GD, Jutooru I, Safe S, Angel-Morales G, Mertens-Talcott SU. The drug resistance suppression induced by curcuminoids in colon cancer SW-480 cells is mediated by reactive oxygen species-induced disruption of the microRNA-27a-ZBTB10-Sp axis. Molecular Nutrition and Food Research. 2013 Sep;57(9):1638-48.
- Bahrami A, Majeed M, Sahebkar A. Curcumin: a potent agent to reverse epithelial-to-mesenchymal transition. Cellular Oncology (Dordrecht). 2019 Aug;42(4):405-421.
- Alschuler LN, Gazella KA. The Definitive Guide to Cancer, 3rd Edition: An Integrative Approach to Prevention, Treatment, and Healing. Berkeley, California: Celestial Arts. 2010; Alschuler LN, Gazella KA. The Definitive Guide to Thriving after Cancer: A Five-Step Integrative Plan to Reduce the Risk of Recurrence and Build Lifelong Health. Berkeley, California: Ten Speed Press. 2013.
- Lemole G, Mehta P, McKee D. After Cancer Care: The Definitive Self-Care Guide to Getting and Staying Well for Patients with Cancer. New York, New York: Rodale, Inc. 2015.
- McKinney N. Naturopathic Oncology, 3rd Edition. Victoria, BC, Canada: Liaison Press. 2016.
- Abrams DI, Weil AT. Integrative Oncology, 2nd Edition. New York, NY: Oxford University Press. 2014.
- Mueller T, Voigt W. Fermented wheat germ extract—nutritional supplement or anticancer drug? Nutrition Journal. 2011 Sep 5;10:89; Yeend T, Robinson K, Lockwood C, McArthur A. The effectiveness of fermented wheat germ extract as an adjunct therapy in the treatment of cancer: a systematic review. JBI Library of Systematic Reviews. 2012;10(42 Suppl):1-12.
- Mueller T, Voigt W. Fermented wheat germ extract—nutritional supplement or anticancer drug? Nutrition Journal. 2011 Sep 5;10:89; Mueller T, Jordan K, Voigt W. Promising cytotoxic activity profile of fermented wheat germ extract (Avemar®) in human cancer cell lines. Journal of Experimental & Clinical Cancer Research. 2011 Apr 16;30(1):42.
- Mueller T, Voigt W. Fermented wheat germ extract—nutritional supplement or anticancer drug? Nutrition Journal. 2011 Sep 5;10:89; Yeend T, Robinson K, Lockwood C, McArthur A. The effectiveness of fermented wheat germ extract as an adjunct therapy in the treatment of cancer: a systematic review. JBI Library of Systematic Reviews. 2012;10(42 Suppl):1-12.
- Zhurakivska K, Troiano G et al. The effects of adjuvant fermented wheat germ extract on cancer cell lines: a systematic review. Nutrients. 2018 Oct 19;10(10):1546.
- Mueller T, Voigt W. Fermented wheat germ extract—nutritional supplement or anticancer drug? Nutrition Journal. 2011 Sep 5;10:89.
- Zhurakivska K, Troiano G et al. The effects of adjuvant fermented wheat germ extract on cancer cell lines: a systematic review. Nutrients. 2018 Oct 19;10(10):1546.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Parmar G, Kaczor T. Textbook of Naturopathic Oncology: A Desktop Guide of Integrative Cancer Care. 1st edition. Canada: Medicatrix Holdings Ltd. 2020.
- Liu C, Li P et al. Advances in the antagonism of epigallocatechin-3-gallate in the treatment of digestive tract tumors. Molecules. 2019 May 3;24(9). pii: E1726; Kumar N, Shibata D, Helm J, Coppola D, Malafa M. Green tea polyphenols in the prevention of colon cancer. Frontiers in Bioscience. 2007 Jan 1;12:2309-15; Liu C, Li P et al. Advances in the antagonism of epigallocatechin-3-gallate in the treatment of digestive tract tumors. Molecules. 2019 May 3;24(9). pii: E1726.
- Shimizu M, Adachi S, Masuda M, Kozawa O, Moriwaki H. Cancer chemoprevention with green tea catechins by targeting receptor tyrosine kinases. Molecular Nutrition & Food Research. 2011 Jun;55(6):832-43.
- Shimizu M, Fukutomi Y et al. Green tea extracts for the prevention of metachronous colorectal adenomas: a pilot study. Cancer Epidemiology, Biomarkers & Prevention. 2008 Nov;17(11):3020-5; Shin CM, Lee DH et al. Green tea extracts for the prevention of metachronous colorectal polyps among patients who underwent endoscopic removal of colorectal adenomas: a randomized clinical trial. Clinical Nutrition. 2018 Apr;37(2):452-458.
- Chen J, Huang XF. The signal pathways in azoxymethane-induced colon cancer and preventive implications. Cancer Biology & Therapy. 2009 Jul;8(14):1313-7.
- Fajardo AM, Piazza GA. Chemoprevention in gastrointestinal physiology and disease. Anti-inflammatory approaches for colorectal cancer chemoprevention. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2015 Jul 15;309(2):G59-70; Ullah MF, Bhat SH et al. Pharmacological intervention through dietary nutraceuticals in gastrointestinal neoplasia. Critical Reviews in Food Science and Nutrition. 2016 Jul 3;56(9):1501-18.
- Ullah MF, Bhat SH et al. Pharmacological intervention through dietary nutraceuticals in gastrointestinal neoplasia. Critical Reviews in Food Science and Nutrition. 2016 Jul 3;56(9):1501-18.
- Ullah MF, Bhat SH et al. Pharmacological intervention through dietary nutraceuticals in gastrointestinal neoplasia. Critical Reviews in Food Science and Nutrition. 2016 Jul 3;56(9):1501-18.
- Hu G, Zhang L, Rong Y, Ni X, Sun Y. Downstream carcinogenesis signaling pathways by green tea polyphenols: a translational perspective of chemoprevention and treatment for cancers. Current Drug Metabolism. 2014 Jan;15(1):14-22.
- Alschuler LN, Gazella KA. The Definitive Guide to Cancer, 3rd Edition: An Integrative Approach to Prevention, Treatment, and Healing. Berkeley, California: Celestial Arts. 2010; Alschuler LN, Gazella KA. The Definitive Guide to Thriving after Cancer: A Five-Step Integrative Plan to Reduce the Risk of Recurrence and Build Lifelong Health. Berkeley, California: Ten Speed Press. 2013.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Lemole G, Mehta P, McKee D. After Cancer Care: The Definitive Self-Care Guide to Getting and Staying Well for Patients with Cancer. New York, New York: Rodale, Inc. 2015.
- Lissoni P, Barni S et al. Decreased toxicity and increased efficacy of cancer chemotherapy using the pineal hormone melatonin in metastatic solid tumour patients with poor clinical status. European Journal of Cancer. 1999 Nov;35(12):1688-92.
- Barni S, Lissoni P et al. A randomized study of low-dose subcutaneous interleukin-2 plus melatonin versus supportive care alone in metastatic colorectal cancer patients progressing under 5-fluorouracil and folates. Oncology. 1995;52:243–245; Lissoni P, Brivio F et al. Neuroimmunomodulation in medical oncology: application of psychoneuroimmunology with subcutaneous low-dose IL-2 and the pineal hormone melatonin in patients with untreatable metastatic solid tumors. Anticancer Research. 2008 Mar-Apr;28(2B):1377-81.
- Cerea G, Vaghi M et al. Biomodulation of cancer chemotherapy for metastatic colorectal cancer: a randomized study of weekly low-dose irinotecan alone versus irinotecan plus the oncostatic pineal hormone melatonin in metastatic colorectal cancer patients progressing on 5-fluorouracil-containing combinations. Anticancer Research. 2003 Mar-Apr;23(2C):1951-4.
- Alschuler LN, Gazella KA. The Definitive Guide to Cancer, 3rd Edition: An Integrative Approach to Prevention, Treatment, and Healing. Berkeley, California: Celestial Arts. 2010; Alschuler LN, Gazella KA. The Definitive Guide to Thriving after Cancer: A Five-Step Integrative Plan to Reduce the Risk of Recurrence and Build Lifelong Health. Berkeley, California: Ten Speed Press. 2013.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Lemole G, Mehta P, McKee D. After Cancer Care: The Definitive Self-Care Guide to Getting and Staying Well for Patients with Cancer. New York, New York: Rodale, Inc. 2015.
- McKinney N. Naturopathic Oncology, 3rd Edition. Victoria, BC, Canada: Liaison Press. 2016.
- Parmar G, Kaczor T. Textbook of Naturopathic Oncology: A Desktop Guide of Integrative Cancer Care. 1st edition. Canada: Medicatrix Holdings Ltd. 2020.
- Friedel WE, Matthes H, Bock PR, Zänker KS. Systematic evaluation of the clinical effects of supportive mistletoe treatment within chemo- and/or radiotherapy protocols and long-term mistletoe application in nonmetastatic colorectal carcinoma: multicenter, controlled, observational cohort study. Journal of the Society for Integrative Oncology. 2009 Fall;7(4):137-45.
- Parmar G, Kaczor T. Textbook of Naturopathic Oncology: A Desktop Guide of Integrative Cancer Care. 1st edition. Canada: Medicatrix Holdings Ltd. 2020.
- Song M, Zhang X et al. Marine ω-3 polyunsaturated fatty acid intake and survival after colorectal cancer diagnosis. Gut. 2017 Oct;66(10):1790-1796.
- Adiamah A, Skořepa P, Weimann A, Lobo DN. The impact of preoperative immune modulating nutrition on outcomes in patients undergoing surgery for gastrointestinal cancer: a systematic review and meta-analysis. Annals of Surgery. 2019 Feb 26.
- de Aguiar Pastore Silva J, Emilia de Souza Fabre M, Waitzberg DL. Omega-3 supplements for patients in chemotherapy and/or radiotherapy: a systematic review. Clinical Nutrition. 2015 Jun;34(3):359-66.
- Cheng J, Ogawa K et al. Increased intake of n-3 polyunsaturated fatty acids elevates the level of apoptosis in the normal sigmoid colon of patients polypectomized for adenomas/tumors. Cancer Letters. 2003 Apr 10;193(1):17-24.
- Cockbain AJ, Volpato M et al. Anticolorectal cancer activity of the omega-3 polyunsaturated fatty acid eicosapentaenoic acid. Gut. 2014 Nov;63(11):1760-8.
- Cockbain AJ, Volpato M et al. Anticolorectal cancer activity of the omega-3 polyunsaturated fatty acid eicosapentaenoic acid. Gut. 2014 Nov;63(11):1760-8.
- Courtney ED, Matthews S et al. Eicosapentaenoic acid (EPA) reduces crypt cell proliferation and increases apoptosis in normal colonic mucosa in subjects with a history of colorectal adenomas. International Journal of Colorectal Disease. 2007 Jul;22(7):765-76.
- Alschuler LN, Gazella KA. The Definitive Guide to Cancer, 3rd Edition: An Integrative Approach to Prevention, Treatment, and Healing. Berkeley, California: Celestial Arts. 2010; Alschuler LN, Gazella KA. The Definitive Guide to Thriving after Cancer: A Five-Step Integrative Plan to Reduce the Risk of Recurrence and Build Lifelong Health. Berkeley, California: Ten Speed Press. 2013.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Lemole G, Mehta P, McKee D. After Cancer Care: The Definitive Self-Care Guide to Getting and Staying Well for Patients with Cancer. New York, New York: Rodale, Inc. 2015.
- McKinney N. Naturopathic Oncology, 3rd Edition. Victoria, BC, Canada: Liaison Press. 2016.
- Parmar G, Kaczor T. Textbook of Naturopathic Oncology: A Desktop Guide of Integrative Cancer Care. 1st edition. Canada: Medicatrix Holdings Ltd. 2020.
- Howells LM, Berry DP et al. Phase I randomized, double-blind pilot study of micronized resveratrol (SRT501) in patients with hepatic metastases--safety, pharmacokinetics, and pharmacodynamics. Cancer Prevention Research (Philadelphia, Pa.). 2011;4(9):1419-1425.
- Patel KR, Brown VA et al. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Research. 2010 Oct 1;70(19):7392-9.
- Schneider Y, Duranton B et al. Resveratrol inhibits intestinal tumorigenesis and modulates host-defense-related gene expression in an animal model of human familial adenomatous polyposis.Nutrition and Cancer. 2001;39(1):102-7; Cui X, Jin Y et al. Resveratrol suppresses colitis and colon cancer associated with colitis. Cancer Prevention Research (Philadelphia). 2010 Apr;3(4):549-59.
- Santandreu FM, Valle A, Oliver J, Roca P. Resveratrol potentiates the cytotoxic oxidative stress induced by chemotherapy in human colon cancer cells. Cell Physiology and Biochemistry. 2011;28(2):219-28.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Lemole G, Mehta P, McKee D. After Cancer Care: The Definitive Self-Care Guide to Getting and Staying Well for Patients with Cancer. New York, New York: Rodale, Inc. 2015.
- McKinney N. Naturopathic Oncology, 3rd Edition. Victoria, BC, Canada: Liaison Press. 2016.
- Tanaka S, Haruma K, Yoshihara M, Kajiyama G, Kira K, Amagase H, Chayama K. Aged garlic extract has potential suppressive effect on colorectal adenomas in humans. Journal of Nutrition. 2006 Mar;136(3 Suppl):821S-826S.
- Chen M, May BH, Zhou IW, Xue CC, Zhang AL. Meta-analysis of oxaliplatin-based chemotherapy combined with traditional medicines for colorectal cancer: contributions of specific plants to tumor response. Integrative Cancer Therapies. 2016;15(1):40–59.
- Huang S, Peng W et al. Kangai injection, a traditional Chinese medicine, improves efficacy and reduces toxicity of chemotherapy in advanced colorectal cancer patients: a systematic review and meta-analysis. Evidence-based Complementary and Alternative Medicine. 2019 Jul 15;2019:8423037.
- Yu R, Wu X, Jia L, Lou Y. Effect of Chinese herbal compound LC09 on patients with capecitabine-associated hand-foot syndrome: a randomized, double-blind, and parallel-controlled trial. Integrative Cancer Therapies. Jan-Dec 2020;19:1534735420928466.
- Chen WT, Yang TS et al. Effectiveness of a novel herbal agent MB-6 as a potential adjunct to 5-fluoracil-based chemotherapy in colorectal cancer. Nutrition Research. 2014 Jul;34(7):585-94.
- Chen M, May BH, Zhou IW, Xue CC, Zhang AL. Meta-analysis of oxaliplatin-based chemotherapy combined with traditional medicines for colorectal cancer: contributions of specific plants to tumor response. Integrative Cancer Therapies. 2016;15(1):40–59.
- Zhang T, Yang YF et al. Efficacy and safety of Quxie Capsule () in metastatic colorectal cancer: a double-blind randomized placebo controlled trial. Chinese Journal of Integrative Medicine. 2018;24(3):171–177; Zhang T, Yang YF et al. Efficacy of quxie capsule in metastatic colorectal cancer: update of a double-blind, randomized, placebo controlled trial. Journal of Clinical Oncology. 2019;37(15_supp); Yang YF, Chen ZX, Xu Y. [Randomized controlled study on effect of Quxie Capsule on the median survival time and quality of life in patients with advanced colorectal carcinoma] [Article in Chinese]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2008;28(2):111–114.
- Yang YF, Xu Y, Wu Y. Clinical randomized double-blinded controlled study on Quxie Capsule in reducing post-operational relapse and metastasis of colorectal cancer] [Article in Chinese]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2007;27(10):879–882.
- Chen D, Yang Y, Yang P. Quxie Capsule inhibits colon tumor growth partially through foxo1-mediated apoptosis and immune modulation. Integrative Cancer Therapies. 2019;18:1534735419846377.
- Yehia R, Saleh S, El Abhar H, Saad AS, Schaalan M. L-Carnosine protects against oxaliplatin-induced peripheral neuropathy in colorectal cancer patients: a perspective on targeting Nrf-2 and NF-κB pathways. Toxicology and Applied Pharmacology. 2019;365:41-50.
- Gupta N, Saleem A et al. Carbogen and nicotinamide increase blood flow and 5-fluorouracil delivery but not 5-fluorouracil retention in colorectal cancer metastases in patients. Clinical Cancer Research. 2006;12(10):3115–3123.
- El Halabi I, Bejjany R et al. Ascorbic acid in colon cancer: from the basic to the clinical applications. International Journal of Moleculal Sciences. 2018;19(9):2752.
- Murata A, Morishige F, Yamaguchi H. Prolongation of survival times of terminal cancer patients by administration of large doses of ascorbate. International Journal for Vitam and Nutrition Research. Supplement. 1982;23:103-13.
- El Halabi I, Bejjany R et al. Ascorbic acid in colon cancer: from the basic to the clinical applications. International Journal of Moleculal Sciences. 2018;19(9):2752
- Yang LC, Hsieh CC, Lu TJ, Lin WC. Structurally characterized arabinogalactan from Anoectochilus formosanus as an immuno-modulator against CT26 colon cancer in BALB/c mice. Phytomedicine. 2014 Apr 15;21(5):647-55.
- Hagmar B, Ryd W, Skomedal H. Arabinogalactan blockade of experimental metastases to liver by murine hepatoma. Invasion Metastasis. 1991;11(6):348-55.
- Derry MM, Raina K, Agarwal R, Agarwal C. Characterization of azoxymethane-induced colon tumor metastasis to lung in a mouse model relevant to human sporadic colorectal cancer and evaluation of grape seed extract efficacy. Experimental and Toxicologic Pathology. 2014 Aug;66(5-6):235-42.
- Kaur M, Singh RP, Gu M, Agarwal R, Agarwal C. Grape seed extract inhibits in vitro and in vivo growth of human colorectal carcinoma cells. Clinical Cancer Research. 2006 Oct 15;12(20 Pt 1):6194-202.
- Cheah KY, Howarth GS, Bastian SE. Grape seed extract dose-responsively decreases disease severity in a rat model of mucositis; concomitantly enhancing chemotherapeutic effectiveness in colon cancer cells. PLoS One. 2014 Jan 21;9(1):e85184.
- Kim DJ, Shin DH et al. Chemoprevention of colon cancer by Korean food plant components. Mutation Research. 2003 Feb-Mar;523-524:99-107.
- Maneikyte J, Bausys A et al. Dietary glycine decreases both tumor volume and vascularization in a combined colorectal liver metastasis and chemotherapy model. International Journal of Biological Sciences. 2019 Jun 4;15(8):1582-1590.
- Ranji P, Akbarzadeh A, Rahmati-Yamchi M. Associations of probiotics with vitamin D and leptin receptors and their effects on colon cancer. Asian Pacific Journal of Cancer Prevention. 2015;16(9):3621-7; Shang M, Sun J. Vitamin D/VDR, probiotics, and gastrointestinal diseases. Current Medicinal Chemistry. 2017;24(9):876-887.
- Albandar HJ, Markert R, Agrawal S. The relationship between aspirin use and mortality in colorectal cancer. Journal of Gastrointestinal Oncology. 2018 Dec;9(6):1133-1137; Bains SJ, Mahic M et al. Aspirin as secondary prevention in patients with colorectal cancer: an unselected population-based study. Journal of Clinical Oncology. 2016 Jul 20;34(21):2501-8.
- Li P, Wu H et al. Aspirin use after diagnosis but not prediagnosis improves established colorectal cancer survival: a meta-analysis. Gut. 2015 Sep;64(9):1419-25.
- Figueiredo JC, Jacobs EJ, Newton CC, Guinter MA, Cance WG, Campbell PT. Associations of aspirin and non-aspirin non-steroidal anti-inflammatory drugs with colorectal cancer mortality after diagnosis. NCI: Journal of the National Cancer Institute. 2021 Feb 2:djab008.
- Frouws MA, Reimers MS et al. The influence of BRAF and KRAS mutation status on the association between aspirin use and survival after colon cancer diagnosis.PLoS One. 2017 Jan 26;12(1):e0170775.
- Durko L, Malecka-Panas E et al. Lifestyle modifications and colorectal cancer. Current Colorectal Cancer Reports. 2014;10:45-54.
- Loomans-Kropp HA, Pinsky P, Cao Y, Chan AT, Umar A. Association of aspirin use with mortality risk among older adult participants in the prostate, lung, colorectal, and ovarian cancer screening trial. JAMA Network Open. 2019 Dec 2;2(12):e1916729.
- Mohammed A, Yarla NS, Madka V, Rao CV. Clinically relevant anti-inflammatory agents for chemoprevention of colorectal cancer: new perspectives. International Journal of Molecular Sciences. 2018 Aug 8;19(8). pii: E2332.
- Fong W, To KKW. Drug repurposing to overcome resistance to various therapies for colorectal cancer. Cellular and Molecular Life Sciences. 2019 Sep;76(17):3383-3406.
- Restivo A, Cocco IM et al. Aspirin as a neoadjuvant agent during preoperative chemoradiation for rectal cancer. British Journal of Cancer. 2015 Oct 20;113(8):1133-9; Elwood PC, Morgan G et al. Aspirin in the treatment of cancer: reductions in metastatic spread and in mortality: a systematic review and meta-analyses of published studies. PLoS One. 2016 Apr 20;11(4):e0152402.
- Fong W, To KKW. Drug repurposing to overcome resistance to various therapies for colorectal cancer. Cellular and Molecular Life Sciences. 2019;76(17):3383–3406.
- Fong W, To KKW. Drug repurposing to overcome resistance to various therapies for colorectal cancer. Cellular and Molecular Life Sciences. 2019 Sep;76(17):3383-3406.
- McNeil JJ, Gibbs P et al. Effect of aspirin on cancer incidence and mortality in older adults. Journal of the National Cancer Institute. 2020;djaa114.
- Wakeman C, Keenan J et al. Chemoprevention of colorectal neoplasia. ANZ Journal of Surgery. 2017 Dec;87(12):E228-E232.
- Gash KJ, Chambers AC, Cotton DE, Williams AC, Thomas MG. Potentiating the effects of radiotherapy in rectal cancer: the role of aspirin, statins and metformin as adjuncts to therapy. British Journal of Cancer. 2017 Jul 11;117(2):210-219.
- Restivo A, Cocco IM et al. Aspirin as a neoadjuvant agent during preoperative chemoradiation for rectal cancer. British Journal of Cancer. 2015 Oct 20;113(8):1133-9.
- Hardie C, Jung Y, Jameson M. Effect of statin and aspirin use on toxicity and pathological complete response rate of neo-adjuvant chemoradiation for rectal cancer. Asia-Pacific Journal of Clinical Oncology. 2016 Jun;12(2):167-73.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Chang R. Beyond the Magic Bullet: The Anti-Cancer Cocktail. New York: Square One Publishers. 2012.
- Lévi F, Zidani R, Misset JL. Randomised multicentre trial of chronotherapy with oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer. International Organization for Cancer Chronotherapy. Lancet. 1997 Sep 6;350(9079):681-6.
- Innominato PF, Roche VP et al. The circadian timing system in clinical oncology. Annals of Medicine. 2014 Jun;46(4):191-207; Giacchetti S, Dugué PA et al.Sex moderates circadian chemotherapy effects on survival of patients with metastatic colorectal cancer: a meta-analysis. Annals of Oncology. 2012 Dec;23(12):3110-6; Lévi F. Chronotherapeutics: the relevance of timing in cancer therapy. Cancer Causes & Control. 2006 May;17(4):611-21; Lévi F, Focan C et al. Implications of circadian clocks for the rhythmic delivery of cancer therapeutics. Advanced Drug Delivery Reviews. 2007;59(9-10):1015–1035; Liao C, Li J, Bin Q, Cao Y, Gao F. Chronomodulated chemotherapy versus conventional chemotherapy for advanced colorectal cancer: a meta-analysis of five randomized controlled trials. International Journal of Colorectal Disease. 2010 Mar;25(3):343-50; Mormont MC, Waterhouse J et al. Marked 24-h rest/activity rhythms are associated with better quality of life, better response, and longer survival in patients with metastatic colorectal cancer and good performance status. Clinical Cancer Research. 2000;6(8):3038–3045.
- Lis CG, Grutsch JF et al. Circadian timing in cancer treatment: the biological foundation for an integrative approach. Integrative Cancer Therapies. 2003 Jun;2(2):105-11.
- Lévi F, Zidani R, Misset JL. Randomised multicentre trial of chronotherapy with oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer. International Organization for Cancer Chronotherapy. Lancet. 1997;350(9079):681–686.
- Lévi FA, Zidani R et al. Chronomodulated versus fixed-infusion-rate delivery of ambulatory chemotherapy with oxaliplatin, fluorouracil, and folinic acid (leucovorin) in patients with colorectal cancer metastases: a randomized multi-institutional trial. Journal of the National Cancer Institute. 1994 Nov 2;86(21):1608-17.
- Giacchetti S, Bjarnason G et al. First line infusion of 5-fluorouracil, leucovorin and oxaliplatin for metastatic colorectal cancer: 4-day chronomodulated (FFL4–10) versus 2-day FOLFOX2. A multicenter randomized phase III trial of the Chronotherapy Group of the European Organization for Research and Treatment of Cancer (EORTC 05963). Journal of Clinical Oncology. 2004 Jul 15;22(14_suppl):3526-3526.
- Lévi F, Focan C et al. Implications of circadian clocks for the rhythmic delivery of cancer therapeutics. Advanced Drug Delivery Reviews. 2007;59(9-10):1015–1035.
- Lévi F, Karaboué A et al. Cetuximab and circadian chronomodulated chemotherapy as salvage treatment for metastatic colorectal cancer (mCRC): safety, efficacy and improved secondary surgical resectability. Cancer Chemotherapy and Pharmacology. 2011;67(2):339–348; Garufi C, Torsello A et al. Cetuximab plus chronomodulated irinotecan, 5-fluorouracil, leucovorin and oxaliplatin as neoadjuvant chemotherapy in colorectal liver metastases: POCHER trial. British Journal of Cancer. 2010 Nov 9;103(10):1542-7; Garufi C, Torsello A et al. Cetuximab plus chronomodulated irinotecan, 5-fluorouracil, leucovorin and oxaliplatin as neoadjuvant chemotherapy in colorectal liver metastases: POCHER trial. British Journal of Cancer. 2010 Nov 9;103(10):1542-7.
- Block KI, Block PB et al. Making circadian cancer therapy practical. Integrative Cancer Therapies. 2009 Dec;8(4):371-86.
- Lévi F, Focan C et al.Implications of circadian clocks for the rhythmic delivery of cancer therapeutics. Advanced Drug Delivery Reviews. 2007;59(9-10):1015–1035.
- Jacobs BA, Deenen MJ et al. Pronounced between-subject and circadian variability in thymidylate synthase and dihydropyrimidine dehydrogenase enzyme activity in human volunteers. British Journal of Clinical Pharmacology. 2016 Sep;82(3):706-16.
- Baker D. Application of chronotherapy to the treatment of cancer: can changing the timing of drug administration influence efficacy and toxicity? Advances in Pharmacy. 2004 Jul;2(3):222-228.
- Lee JH, Kim TI et al. The effects of metformin on the survival of colorectal cancer patients with diabetes mellitus. International Journal of Cancer. 2012;131:752–759; Yang IP, Miao ZF et al. High blood sugar levels but not diabetes mellitus significantly enhance oxaliplatin chemoresistance in patients with stage III colorectal cancer receiving adjuvant FOLFOX6 chemotherapy. Therapeutic Advanced in Medical Oncology. 2019 Aug 20;11:1758835919866964.
- Mafiana RN, Al-Kindi MS, Mafiana N, Al Lawati AS, Al Moundhri M. Impact of Metabolic syndrome diagnosis and its treatment on survival of colorectal cancer patients. Journal of Cancer Epidemiology. 2019 Apr 21;2019:6527457.
- Gash KJ, Chambers AC, Cotton DE, Williams AC, Thomas MG. Potentiating the effects of radiotherapy in rectal cancer: the role of aspirin, statins and metformin as adjuncts to therapy. British Journal of Cancer. 2017 Jul 11;117(2):210-219.
- Bragagnoli A, Araujo R et al. Final results of a phase II of metformin plus irinotecan for refractory colorectal cancer. Journal of Clinical Oncology. 2018;36(15_suppl):e15527-e15527.
- Miranda VC, Braghiroli MI et al. Phase 2 trial of metformin combined with 5-fluorouracil in patients with refractory metastatic colorectal cancer. Clinical Colorectal Cancer. 2016 Dec;15(4):321-328.e1.
- Yang IP, Miao ZF et al. High blood sugar levels but not diabetes mellitus significantly enhance oxaliplatin chemoresistance in patients with stage III colorectal cancer receiving adjuvant FOLFOX6 chemotherapy. Therapeutic Advanced in Medical Oncology. 2019 Aug 20;11:1758835919866964.
- Coyle C, Cafferty FH, Vale C, Langley RE. Metformin as an adjuvant treatment for cancer: a systematic review and meta-analysis. Annals of Oncology. 2016;27(12):2184-2195.
- Eikawa S, Nishida M et al. Immune-mediated antitumor effect by type 2 diabetes drug, metformin. Proceedings of the National Academy of Sciences of the United States of America. 2015 Feb 10;112(6):1809-14.
- Cha JH, Yang WH et al. Metformin promotes antitumor immunity via endoplasmic-reticulum-associated degradation of PD-L1. Molecular Cell. 2018 Aug 16;71(4):606-620.e7.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Mei Z, Liang M et al. Effects of statins on cancer mortality and progression: a systematic review and meta-analysis of 95 cohorts including 1,111,407 individuals. International Journal of Cancer. 2017 Mar 1;140(5):1068-1081; He Y, Li X et al. Statins and multiple noncardiovascular outcomes: umbrella review of meta-analyses of observational studies and randomized controlled trials. Annals of Internal Medicine. 2018 Oct 16;169(8):543-553.
- Yokomichi H, Nagai A et al. Statin use and all-cause and cancer mortality: BioBank Japan cohort. Journal of Epidemiology. 2017 Mar;27(3S):S84-S91.
- Li Y, He X, Ding Y, Chen H, Sun L. Statin uses and mortality in colorectal cancer patients: An updated systematic review and meta-analysis. Cancer Medicine. 2019;8(6):3305–3313; Zhong S, Zhang X et al. Statin use and mortality in cancer patients: systematic review and meta-analysis of observational studies. Cancer Treatment Reviews. 2015 Jun;41(6):554-67; Ling Y, Yang L et al. Prognostic significance of statin use in colorectal cancer: a systematic review and meta-analysis. Medicine (Baltimore). 2015 Jun;94(25):e908; Cai H, Zhang G, Wang Z, Luo Z, Zhou X. Relationship between the use of statins and patient survival in colorectal cancer: a systematic review and meta-analysis. PLoS One. 2015 Jun 1;10(6):e0126944; Gray RT, Coleman HG, Hughes C, Murray LJ, Cardwell CR. Statin use and survival in colorectal cancer: results from a population-based cohort study and an updated systematic review and meta-analysis. Cancer Epidemiology. 2016 Dec;45:71-81.
- Mafiana RN, Al-Kindi MS, Mafiana N, Al Lawati AS, Al Moundhri M. Impact of metabolic syndrome diagnosis and its treatment on survival of colorectal cancer patients. Journal of Cancer Epidemiology. 2019;2019:6527457.
- Joo MK, Park JJ, Chun HJ. Additional benefits of routine drugs on gastrointestinal cancer: statins, metformin, and proton pump inhibitors. Digestive Diseases. 2018;36(1):1-14.
- Qu B, Qu H. The influence of statins on risk and patient survival in colorectal cancer. Journal of Clinical Gastroenterology. 2019;53(9):699–701.
- Fransgaard T, Hallas J, Thygesen LC, Gögenur I. Association of statin use and oncological outcomes after neoadjuvant radiotherapy in patients with rectal cancer. Anticancer Research. 2019;39(4):2177–2182.
- Rutledge BP, Desai P et al. The association between statins and colorectal cancer stage in the Women's Health Initiative. Molecular and Clinical Oncology. 2019;11(3):252–258.
- Coogan PF, Smith J, Rosenberg L. Statin use and risk of colorectal cancer. Journal of the National Cancer Institute. 2007;99(1):32–40.
- Gash KJ, Chambers AC, Cotton DE, Williams AC, Thomas MG. Potentiating the effects of radiotherapy in rectal cancer: the role of aspirin, statins and metformin as adjuncts to therapy. British Journal of Cancer. 2017 Jul 11;117(2):210-219.
- Qu B, Qu H. The influence of statins on risk and patient survival in colorectal cancer. Journal of Clinical Gastroenterology. 2019;53(9):699–701.
- Qu B, Qu H. The influence of statins on risk and patient survival in colorectal cancer. Journal of Clinical Gastroenterology. 2019;53(9):699–701; Krens LL, Simkens LH, Baas JM, et al. Statin use is not associated with improved progression free survival in cetuximab treated KRAS mutant metastatic colorectal cancer patients: results from the CAIRO2 study. PLoS One. 2014;9(11):e112201.
- Fong W, To KKW. Drug repurposing to overcome resistance to various therapies for colorectal cancer. Cellular and Molecular Life Sciences. 2019;76(17):3383–3406.
- Fong W, To KKW. Drug repurposing to overcome resistance to various therapies for colorectal cancer. Cellular and Molecular Life Sciences. 2019;76(17):3383–3406.
- Ahn KS, Sethi G, Aggarwal BB. Reversal of chemoresistance and enhancement of apoptosis by statins through down-regulation of the NF-kappaB pathway. Biochemical pharmacology. 2008 Feb 15;75(4):907-13.
- Agarwal B, Bhendwal S et al. Lovastatin augments apoptosis induced by chemotherapeutic agents in colon cancer cells. Clinical Cancer Research. 1999;5(8):2223–2229.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Krishna S, Ganapathi S et al. A randomised, double blind, placebo-controlled pilot study of oral artesunate therapy for colorectal cancer. EBioMedicine. 2014 Nov 15;2(1):82-90.
- Raffetin A, Bruneel F et al. Use of artesunate in non-malarial indications. Médecine et Maladies Infectieuses. 2018;48(4):238–249.
- Wang LL, Kong L, Liu H, et al. Design and synthesis of novel artemisinin derivatives with potent activities against colorectal cancer in vitro and in vivo. European Journal of Medicinal Chemistry. 2019;182:111665.
- Yao Z, Bhandari A, Wang Y, et al. Dihydroartemisinin potentiates antitumor activity of 5-fluorouracil against a resistant colorectal cancer cell line. Biochemical and Biophysical Research Communications. 2018;501(3):636–642.
- Kang XJ, Wang HY, Peng HG, et al. Codelivery of dihydroartemisinin and doxorubicin in mannosylated liposomes for drug-resistant colon cancer therapy. Acta Pharmacol Sin. 2017;38(6):885–896.
- Lee DH, Hasanuzzaman M et al. 10-phenylpyrazole artemisinin is a novel P-glycoprotein inhibitor that suppresses the overexpression and function of P-glycoprotein. Current Pharmaceutical Design. 2018;24(46):5590–5597.
- Parmar G, Kaczor T. Textbook of Naturopathic Oncology: A Desktop Guide of Integrative Cancer Care. 1st edition. Canada: Medicatrix Holdings Ltd. 2020.
- Losurdo G, Principi M et al. Histamine and histaminergic receptors in colorectal cancer: from basic science to evidence-based medicine. Anti-Cancer Agents in Medicinal Chemistry. 2018;18(1):15–20.
- Deva S, Jameson M. Histamine type 2 receptor antagonists as adjuvant treatment for resected colorectal cancer. Cochrane Database of Systematic Reviews. 2012;(8):CD007814; Pantziarka P, Bouche G, Meheus L, Sukhatme V, Sukhatme VP. Repurposing drugs in oncology (ReDO)—cimetidine as an anti-cancer agent. Ecancermedicalscience. 2014;8:485; Kelly MD, King J et al. Randomized trial of preoperative cimetidine in patients with colorectal carcinoma with quantitative assessment of tumor-associated lymphocytes. Cancer. 1999 Apr 15;85(8):1658-63.
- Svendsen LB, Ross C et al. Cimetidine as an adjuvant treatment in colorectal cancer. A double-blind, randomized pilot study. Diseases of the Colon and Rectum. 1995 May;38(5):514-8; Jameson MB, Michael Arendse, Pillai A et al. Final analysis of a randomized placebo-controlled double-blind phase II trial of perioperative cimetidine (CIM) in early colorectal cancer (CRC). Journal of Clinical Oncology. 2018;36(15_suppl):e15678-e15678; Yoshimatsu K, Ishibashi K et al. [Can the survival of patients with recurrent disease after curative resection of colorectal cancer be prolonged by the administration of cimetidine?] [Article in Japanese]. Gan To Kagaku Ryoho. 2006 Nov;33(12):1730-2.
- Pantziarka P, Bouche G, Meheus L, Sukhatme V, Sukhatme VP. Repurposing drugs in oncology (ReDO)—cimetidine as an anti-cancer agent. Ecancermedicalscience. 2014;8:485.
- Lin CY, Bai DJ, Yuan HY, et al. Perioperative cimetidine administration promotes peripheral blood lymphocytes and tumor infiltrating lymphocytes in patients with gastrointestinal cancer: results of a randomized controlled clinical trial. World Journal of Gastroenterology. 2004;10(1):136–142.
- Losurdo G, Principi M et al. Histamine and histaminergic receptors in colorectal cancer: from basic science to evidence-based medicine. Anti-Cancer Agents in Medicinal Chemistry. 2018;18(1):15–20; Pantziarka P, Bouche G, Meheus L, Sukhatme V, Sukhatme VP. Repurposing drugs in oncology (ReDO)—cimetidine as an anti-cancer agent. Ecancermedicalscience. 2014;8:485.
- Losurdo G, Principi M et al. Histamine and histaminergic receptors in colorectal cancer: from basic science to evidence-based medicine. Anti-Cancer Agents in Medicinal Chemistry. 2018;18(1):15–20; Pantziarka P, Bouche G, Meheus L, Sukhatme V, Sukhatme VP. Repurposing drugs in oncology (ReDO)—cimetidine as an anti-cancer agent. Ecancermedicalscience. 2014;8:485.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Chang R. Beyond the Magic Bullet: The Anti-Cancer Cocktail. New York: Square One Publishers. 2012.
- Fong W, To KKW. Drug repurposing to overcome resistance to various therapies for colorectal cancer. Cellular and Molecular Life Sciences. 2019;76(17):3383–3406; Mahalingam D, Mita M et al. Combined autophagy and HDAC inhibition: a phase I safety, tolerability, pharmacokinetic, and pharmacodynamic analysis of hydroxychloroquine in combination with the HDAC inhibitor vorinostat in patients with advanced solid tumors. Autophagy. 2014;10(8):1403–1414.
- Sasaki K, Tsuno NH et al. Chloroquine potentiates the anti-cancer effect of 5-fluorouracil on colon cancer cells. BMC Cancer. 2010;10:370.
- Fong W, To KKW. Drug repurposing to overcome resistance to various therapies for colorectal cancer. Cellular and Molecular Life Sciences. 2019;76(17):3383–3406; Marinković M, Šprung M, Buljubašić M, Novak I. Autophagy modulation in cancer: current knowledge on action and therapy. Oxidative Medicine and Cellular Longevity. 2018;2018:8023821; Schonewolf CA, Mehta M, Schiff D, et al. Autophagy inhibition by chloroquine sensitizes HT-29 colorectal cancer cells to concurrent chemoradiation. World Journal of Gastrointestinal Oncology. 2014;6(3):74–82.
- Gartner EM, Griffith KA et al. A pilot trial of the anti-angiogenic copper lowering agent tetrathiomolybdate in combination with irinotecan, 5-flurouracil, and leucovorin for metastatic colorectal cancer. Investigational New Drugs. 2009;27(2):159–165.
- Khan G, Merajver S. Copper chelation in cancer therapy using tetrathiomolybdate: an evolving paradigm. Expert Opinion on Investigational Drugs. 2009;18(4):541–548; Brewer GJ. Anticopper therapy against cancer and diseases of inflammation and fibrosis. Drug Discovery Today. 2005;10(16):1103–1109.
- Fatfat M, Merhi RA et al. Copper chelation selectively kills colon cancer cells through redox cycling and generation of reactive oxygen species. BMC Cancer. 2014;14:527.
- Baldari S, Di Rocco G et al. Effects of copper chelation on BRAFV600E positive colon carcinoma cells. Cancers (Basel). 2019;11(5):659.
- Rolim PM, Fidelis GP et al. Phenolic profile and antioxidant activity from peels and seeds of melon (Cucumis melo L. var. reticulatus) and their antiproliferative effect in cancer cells. Brazilian Journal of Medical and Biological Research. 2018;51(4):e6069.
- Yu N, Zhu H et al. Combination of Fe/Cu -chelators and docosahexaenoic acid: an exploration for the treatment of colorectal cancer. Oncotarget. 2017;8(31):51478–51491.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Parmar G, Kaczor T. Textbook of Naturopathic Oncology: A Desktop Guide of Integrative Cancer Care. 1st edition. Canada: Medicatrix Holdings Ltd. 2020.
- Meyn RE, Krishnan S, Skinner HD. Everything old is new again: using nelfinavir to radiosensitize rectal cancer. Clinical Cancer Research. 2016;22(8):1834–1836; Hill EJ, Roberts C et al. Clinical trial of oral nelfinavir before and during radiation therapy for advanced rectal cancer. Clinical Cancer Research. 2016;22(8):1922–1931.
- Buijsen J, Lammering G, Jansen RL, et al. Phase I trial of the combination of the Akt inhibitor nelfinavir and chemoradiation for locally advanced rectal cancer. Radiotherapy & Oncology. 2013;107(2):184–188.
- Bernstein WB, Dennis PA. Repositioning HIV protease inhibitors as cancer therapeutics. Current Opinion in HIV and AIDS. 2008;3(6):666–675
- Gills JJ, Lopiccolo J, Dennis PA. Nelfinavir, a new anti-cancer drug with pleiotropic effects and many paths to autophagy. Autophagy. 2008;4(1):107–109.
- Meyerhardt JA, Shi Q. Effect of celecoxib vs placebo added to standard adjuvant therapy on disease-free survival among patients with stage III colon cancer: the CALGB/SWOG 80702 (Alliance) randomized clinical trial. JAMA. 2021 Apr 6;325(13):1277-1286.
- Fong W, To KKW. Drug repurposing to overcome resistance to various therapies for colorectal cancer. Cellular and Molecular Life Sciences. 2019;76(17):3383–3406.
- Fong W, To KKW. Drug repurposing to overcome resistance to various therapies for colorectal cancer. Cellular and Molecular Life Sciences. 2019;76(17):3383–3406.
- Fong W, To KKW. Drug repurposing to overcome resistance to various therapies for colorectal cancer. Cellular and Molecular Life Sciences. 2019;76(17):3383–3406
- Fong W, To KKW. Drug repurposing to overcome resistance to various therapies for colorectal cancer. Cellular and Molecular Life Sciences. 2019;76(17):3383–3406.
- Pantziarka P, Sukhatme V, Bouche G, Meheus L, Sukhatme VP. Repurposing Drugs in Oncology (ReDO)-diclofenac as an anti-cancer agent. Ecancermedicalscience. 2016;10:610.
- Cohen EE, Sharma MR et al. A phase I study of sirolimus and bevacizumab in patients with advanced malignancies. European Journal of Cancer. 2011;47(10):1484–1489.
- Buck E, Eyzaguirre A et al. Rapamycin synergizes with the epidermal growth factor receptor inhibitor erlotinib in non-small-cell lung, pancreatic, colon, and breast tumors. Molecular Cancer Therapeutics. 2006;5(11):2676–2684.
- Bader JE, Enos RT et al. Macrophage depletion using clodronate liposomes decreases tumorigenesis and alters gut microbiota in the AOM/DSS mouse model of colon cancer. American Journal of Physiology—Gastrointestinal and Liver Physiology. 2018;314(1):G22–G31.
- Baranyi M, Rittler D et al. Next generation lipophilic bisphosphonate shows antitumor effect in colorectal cancer in vitro and in vivo. Pathology Oncology Research. 2020;10.1007/s12253-019-00789-9.
- Zhu J, Liu M et al. Zoledronic acid regulates autophagy and induces apoptosis in colon cancer cell line CT26. BioMed Research International. 2017;2017:7203584; Peng Y, Qiu L et al. M4IDP, a zoledronic acid derivative, induces G1 arrest, apoptosis and autophagy in HCT116 colon carcinoma cells via blocking PI3K/Akt/mTOR pathway. Life Sciences. 2017;185:63–72.
- Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Research Reviews. 2017;39:46–58.
- Lévesque S, Pol JG et al. Trial watch: dietary interventions for cancer therapy. Oncoimmunology. 2019;8(7):1591878.
- Di Tano M, Raucci F et al. Synergistic effect of fasting-mimicking diet and vitamin C against KRAS mutated cancers. Nature Communications. 2020;11(1):2332.
- Scheubeck G, Berchtold S et al. Starvation-induced differential virotherapy using an oncolytic measles vaccine virus. Viruses. 2019;11(7):614.
- Sun P, Wang H, He Z, et al. Fasting inhibits colorectal cancer growth by reducing M2 polarization of tumor-associated macrophages. Oncotarget. 2017;8(43):74649–74660.
- Lee BR, Kim HR et al. Enhanced therapeutic treatment of colorectal cancer using surface-modified nanoporous acupuncture needles. Scientific Reports. 2017;7(1):12900.
- Hager ED, Dziambor H et al. Deep hyperthermia with radiofrequencies in patients with liver metastases from colorectal cancer. Anticancer Research. 1999;19(4C):3403-3408.
- Davenport L. CRC with peritoneal metastases: CRS-HIPEC refinements. Medscape Oncology. July 6, 2020. Viewed July 13, 2020.
- Berdov BA, Menteshashvili GZ. Thermoradiotherapy of patients with locally advanced carcinoma of the rectum. International Journal of Hyperthermia. 1990;6(5):881-890.
- Quénet F, Elias D et al; UNICANCER-GI Group and BIG Renape Group. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021 Jan 18:S1470-2045(20)30599-4.
- Hegewisch-Becker S, Gruber Y et al. Whole-body hyperthermia (41.8 C) combined with bimonthly oxaliplatin, high-dose leucovorin and 5-fluorouracil 48-hour continuous infusion in pretreated metastatic colorectal cancer: a phase II study. Annals of Oncology. 2002;13(8):1197–1204.;Hildebrandt B, Drager J et al. Whole-body hyperthermia in the scope of von Ardenne’s systemic cancer multistep therapy (sCMT) combined with chemotherapy in patients with metastatic colorectal cancer: a phase I/II study. International Journal of Hyperthermia. 2004;20(3):317–333.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Care. New York: Bantam Dell. 2009. p. 329.
- Dhanapal R, Saraswathi T, Govind RN. Cancer cachexia. Journal of Oral and Maxillofacial Pathology. 2011 Sep;15(3):257-60.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Care. New York: Bantam Dell. 2009. p. 329.
- Denlinger CS, Barsevick AM et al. The challenges of colorectal cancer survivorship. Journal of the National Comprehensive Cancer Network. 2009 Sep;7(8):883-93; quiz 894.
- Pulsed low-dose pelvic reirradiation safe, provided tangible benefit for patients with few other options. Fox Chase Cancer Center, September 22, 2020. Viewed September 30, 2020; Paly JJ, Deng M et al. Pelvic reirradiation utilizing pulsed low-dose rate radiation therapy. American Journal of Clinical Oncology. 2020 Oct;43(10):748-751.
- Lin S, An X et al. Meta-analysis of astragalus-containing traditional Chinese medicine combined with chemotherapy for colorectal cancer: efficacy and safety to tumor response. Frontiers in Oncology. 2019;9:749; Taixiang W, Munro AJ, Guanjian L. Chinese medical herbs for chemotherapy side effects in colorectal cancer patients. Cochrane Database of Systematic Reviews. 2005;(1):CD004540; Chen M, May BH, Zhou IW, Xue CC, Zhang AL. FOLFOX 4 combined with herbal medicine for advanced colorectal cancer: a systematic review. Phytotherapy Research. 2014;28(7):976–991.
- Chen M, May BH et al. Oxaliplatin-based chemotherapy combined with traditional medicines for neutropenia in colorectal cancer: a meta-analysis of the contributions of specific plants. Critical Reviews in Oncology/Hematology. 2016;105:18–34.
- Huang WC, Kuo KT, Bamodu OA, et al. Astragalus polysaccharide (PG2) ameliorates cancer symptom clusters, as well as improves quality of life in patients with metastatic disease, through modulation of the inflammatory cascade. Cancers (Basel). 2019;11(8).
- Chen MH, May BH, Zhou IW, Zhang AL, Xue CC. Integrative medicine for relief of nausea and vomiting in the treatment of colorectal cancer using oxaliplatin-based chemotherapy: a systematic review and meta-analysis. Phytotherapy Research. 2016;30(5):741–753.
- Abrams DI, Weil AT. Integrative Oncology, 2nd Edition. New York, NY: Oxford University Press. 2014.
- Lin S, An X et al. Meta-analysis of astragalus-containing traditional Chinese medicine combined with chemotherapy for colorectal cancer: efficacy and safety to tumor response. Frontiers in Oncology. 2019;9:749; Taixiang W, Munro AJ, Guanjian L. Chinese medical herbs for chemotherapy side effects in colorectal cancer patients. Cochrane Database of Systematic Reviews. 2005;(1):CD004540.
- Dong J, Liang W et al. Saponins regulate intestinal inflammation in colon cancer and IBD. Pharmacological Research. 2019;144:66–72.
- Chen D, Zhao J, Cong W. Chinese herbal medicines facilitate the control of chemotherapy-induced side effects in colorectal cancer: progress and perspective. Frontiers in Pharmacology. 2018;9:1442.
- Hao J, Zhu X, Bensoussan A. Effects of nonpharmacological interventions in chemotherapy-induced peripheral neuropathy: an overview of systematic reviews and meta-analyses. Integrative Cancer Therapies. Jan-Dec 2020;19:1534735420945027; Noh H, Yoon SW, Park B. A systematic review of herbal medicine for chemotherapy-induced peripheral neuropathy (CIPN). Evidence Based Complementary and Alternative Medicine. 2018 Feb 14;2018:6194184; Wei X, Zhu L, Wang H, Wang C, Deng Q, Li X. Efficacy of traditional Chinese medicines in preventing oxaliplatin-induced peripheral neurotoxicity in cancer patients: a network meta-analysis. Chinese Herb Med. 2017;9(2):161-168; Deng B, Jia L, Cheng Z. Radix astragali-based Chinese herbal medicine for oxaliplatin-induced peripheral neuropathy: a systematic review and meta-analysis. Evidence-based Complementary and Alternative Medicine. 2016;2016:2421876.
- Lin S, An X et al. Meta-analysis of astragalus-containing traditional Chinese medicine combined with chemotherapy for colorectal cancer: efficacy and safety to tumor response. Frontiers in Oncology. 2019;9:749; Taixiang W, Munro AJ, Guanjian L. Chinese medical herbs for chemotherapy side effects in colorectal cancer patients. Cochrane Database of Systematic Reviews. 2005;(1):CD004540; Huang S, Peng W et al. Kangai injection, a traditional Chinese medicine, improves efficacy and reduces toxicity of chemotherapy in advanced colorectal cancer patients: a systematic review and meta-analysis. Evidence-based Complementary and Alternative Medicine. 2019 Jul 15;2019:8423037.
- Panahia Y, Saadat A et al. Antioxidant effects of bioavailability-enhanced curcuminoids in patients with solid tumors: a randomized double-blind placebo-controlled trial. Journal of Functional Foods. 2014 Jan;6:615-622; Panahi Y, Saadat A, Beiraghdar F, Sahebkar A. Adjuvant therapy with bioavailability-boosted curcuminoids suppresses systemic inflammation and improves quality of life in patients with solid tumors: a randomized double-blind placebo-controlled trial. Phytotherapy Research. 2014 Oct;28(10):1461-7.
- Yeung KS, Gubili J, Mao JJ. Herb-drug interactions in cancer care. Oncology (Williston Park). 2018 Oct 15;32(10):516-20; Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS Journal . 2013 Jan;15(1):195-218.
- He ZY, Shi CB et al. Upregulation of p53 expression in patients with colorectal cancer by administration of curcumin. Cancer Invest. 2011 Mar;29(3):208-13; Lagoa R, Silva J, Rodrigues JR, Bishayee A. Advances in phytochemical delivery systems for improved anticancer activity. Biotechnology Advances. 2019 Apr 9. pii: S0734-9750(19)30063-1; Amalraj A, Pius A, Gopi S, Gopi S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—a review. Journal of Traditional and Complementary Medicine. 2016 Jun 15;7(2):205-233; Belcaro G, Hosoi M et al. A controlled study of a lecithinized delivery system of curcumin (Meriva®) to alleviate the adverse effects of cancer treatment. Phytotherapy Research. 2014 Mar;28(3):444-50; Mansouri K, Rasoulpoor S et al. Clinical effects of curcumin in enhancing cancer therapy: a systematic review. BMC Cancer. 2020;20(1):791.
- Lagoa R, Silva J, Rodrigues JR, Bishayee A. Advances in phytochemical delivery systems for improved anticancer activity. Biotechnology Advances. 2019 Apr 9. pii: S0734-9750(19)30063-1; Amalraj A, Pius A, Gopi S, Gopi S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—a review. Journal of Traditional and Complementary Medicine. 2016 Jun 15;7(2):205-233; Belcaro G, Hosoi M et al. A controlled study of a lecithinized delivery system of curcumin (Meriva®) to alleviate the adverse effects of cancer treatment. Phytotherapy Research. 2014 Mar;28(3):444-50; Mansouri K, Rasoulpoor S et al. Clinical effects of curcumin in enhancing cancer therapy: a systematic review. BMC Cancer. 2020;20(1):791; Normando AGC, Menêses AG et al. Effects of turmeric and curcumin on oral mucositis: A systematic review. Phytherapy Research. 2019;33(5):1318-1329; Delavarian Z, Pakfetrat A et al. Oral administration of nanomicelle curcumin in the prevention of radiotherapy-induced mucositis in head and neck cancers. Special Care in Dentistry. 2019;39(2):166-172; Akbari S, Kariznavi E, Jannati M, Elyasi S, Tayarani-Najaran Z. Curcumin as a preventive or therapeutic measure for chemotherapy and radiotherapy induced adverse reaction: a comprehensive review. Food and Chemical Toxicology. 2020 Nov;145:111699.
- Belcaro G, Hosoi M et al. A controlled study of a lecithinized delivery system of curcumin (Meriva®) to alleviate the adverse effects of cancer treatment. Phytotherapy Research. 2014 Mar; 28:444-50.
- Lagoa R, Silva J, Rodrigues JR, Bishayee A. Advances in phytochemical delivery systems for improved anticancer activity. Biotechnology Advances. 2019 Apr 9. pii: S0734-9750(19)30063-1; Amalraj A, Pius A, Gopi S, Gopi S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—a review. Journal of Traditional and Complementary Medicine. 2016 Jun 15;7(2):205-233; Belcaro G, Hosoi M et al. A controlled study of a lecithinized delivery system of curcumin (Meriva®) to alleviate the adverse effects of cancer treatment. Phytotherapy Research. 2014 Mar;28(3):444-50; Mansouri K, Rasoulpoor S et al. Clinical effects of curcumin in enhancing cancer therapy: a systematic review. BMC Cancer. 2020;20(1):791.
- Chen WT, Yang TS et al. Effectiveness of a novel herbal agent MB-6 as a potential adjunct to 5-fluoracil-based chemotherapy in colorectal cancer. Nutrition Research. 2014 Jul;34(7):585-94.
- Alsherbiny MA, Abd-Elsalam WH et al. Ameliorative and protective effects of ginger and its main constituents against natural, chemical and radiation-induced toxicities: a comprehensive review. Food and Chemical Toxicology. 2019 Jan;123:72-97; Saxena R, Rida PC, Kucuk O, Aneja R. Ginger augmented chemotherapy: a novel multitarget nontoxic approach for cancer management. Molecular Nutrition & Food Research. 2016 Jun;60(6):1364-73.
- Yeung KS, Gubili J, Mao JJ. Herb-drug interactions in cancer care. Oncology (Williston Park). 2018 Oct 15;32(10):516-20; Marx W, Ried K et al. Ginger—mechanism of action in chemotherapy-induced nausea and ; vomiting: a review. Critical Reviews in Food Science and Nutrition. 2017 Jan 2;57(1):141-146; Marx W, Kiss N, Isenring L. Is ginger beneficial for nausea and vomiting? An update of the literature. Current Opinion in supportive and palliative care. 2015 jun;9(2):189-95; Chen D, Zhao J, Cong W. Chinese herbal medicines facilitate the control of chemotherapy-induced side effects in colorectal cancer: progress and perspective. Frontiers in Pharmacology. 2018 Dec 7;9:1442; Marx W, McCarthy AL et al. The effect of a standardized ginger extract on chemotherapy-induced nausea-related quality of life in patients undergoing moderately or highly emetogenic chemotherapy: a double blind, randomized, placebo controlled trial. Nutrients. 2017 Aug 12;9(8). pii: E867; Ryan JL, Heckler CE et al. Ginger (Zingiber officinale) reduces acute chemotherapy-induced nausea: a URCC CCOP study of 576 patients. Supportive Care in Cancer. 2012 Jul;20(7):1479-89; Zick SM, Ruffin MT et al. Phase II trial of encapsulated ginger as a treatment for chemotherapy-induced nausea and vomiting. Supportive Care in Cancer. 2009 May;17(5):563-72.
- Wang WS, Lin JK et al. Oral glutamine is effective for preventing oxaliplatin-induced neuropathy in colorectal cancer patients. Oncologist. 2007 Mar;12(3):312-9.
- Vahdat L, Papadopoulos K et al. Reduction of paclitaxel-induced peripheral neuropathy with glutamine. Clinical Cancer Research. 2001 May;7(5):1192-7.
- Brami C, Bao T, Deng G. Natural products and complementary therapies for chemotherapy-induced peripheral neuropathy: a systematic review. Critical Reviews in Oncology/Hematology. 2016 Feb;98:325-34.
- Jolfaie NR, Mirzaie S, Ghiasvand R, Askari G, Miraghajani M. The effect of glutamine intake on complications of colorectal and colon cancer treatment: a systematic review. Journal of Research in Medical Sciences. 2015 Sep;20(9):910-8.
- Sun J, Wang H, Hu H. Glutamine for chemotherapy induced diarrhea: a meta-analysis. Asia Pacific Journal of Clinical Nutrition. 2012;21(3):380-5.
- Li Y, Ping X et al. Clinical trial: prophylactic intravenous alanyl-glutamine reduces the severity of gastrointestinal toxicity induced by chemotherapy--a randomized crossover study. Alimentary Pharmacology & Therapeutics. 2009 Sep 1;30(5):452-8.
- Ogata Y, Ishibashi N et al. Preventive effects of amino-acid-rich elemental diet Elental® on chemotherapy-induced oral mucositis in patients with colorectal cancer: a prospective pilot study. Supportive Care in Cancer. 2016 Feb;24(2):783-789.
- Daniele B, Perrone F et al. Oral glutamine in the prevention of fluorouracil induced intestinal toxicity: a double blind, placebo controlled, randomised trial. Gut. 2001 Jan;48(1):28-33.
- Khemissa F, Mineur L et al. A phase III study evaluating oral glutamine and transforming growth factor-beta 2 on chemotherapy-induced toxicity in patients with digestive neoplasm. Digestive and Liver Disease. 2016 Mar;48(3):327-32.
- Kucuktulu E, Guner A, Kahraman I, Topbas M, Kucuktulu U. The protective effects of glutamine on radiation-induced diarrhea. Supportive Care in Cancer. 2013 Apr;21(4):1071-5.
- Rotovnik Kozjek N, Kompan L et al. Oral glutamine supplementation during preoperative radiochemotherapy in patients with rectal cancer: a randomised double blinded, placebo controlled pilot study. Clinical Nutrition. 2011 Oct;30(5):567-70.
- Vidal-Casariego A, Calleja-Fernández A et al. Efficacy of glutamine in the prevention of acute radiation enteritis: a randomized controlled trial. JPEN. Journal of Parenteral and Enteral Nutrition. 2014 Feb;38(2):205-13.
- Block KI, Block PB, Gyllenhaal C. Integrative treatment for colorectal cancer: a comprehensive approach. Journal of Alternative and Complementary Medicine. 2018;24(9-10):890–901.
- Kouhi Habibi N, Shabestani Monfared A et al. The protective effects of melatonin on blood cell counts of rectal cancer patients following radio-chemotherapy: a randomized controlled trial. Clinical and Translational Oncology. 2019;21(6):745-752.
- Lissoni P. Is there a role for melatonin in supportive care? Supportive Care in Cancer. 2002 Mar;10(2):110-6; Lissoni P, Barni S et al. Decreased toxicity and increased efficacy of cancer chemotherapy using the pineal hormone melatonin in metastatic solid tumour patients with poor clinical status. European Journal of Cancer. 1999;35(12):1688-1692; Lissoni P, Tancini G et al. Treatment of cancer chemotherapy-induced toxicity with the pineal hormone melatonin. Supportive Care in Cancer. 1997;5(2):126-129.
- Lissoni P, Brivio F et al. Immune effects of preoperative immunotherapy with high-dose subcutaneous interleukin-2 versus neuroimmunotherapy with low-dose interleukin-2 plus the neurohormone melatonin in gastrointestinal tract tumor patients. Journal of Biological Regulators and Homeostatic Agents. 1995;9(1):31-33.
- de Aguiar Pastore Silva J, de Souza Fabre ME, Waitzberg DL. Omega-3 supplements for patients in chemotherapy and/or radiotherapy: a systematic review. Clinical Nutrition. 2015 Jun;34(3):359-66.
- Silva Jde A, Trindade EB et al. Fish oil supplement alters markers of inflammatory and nutritional status in colorectal cancer patients. Nutrition and Cancer. 2012;64(2):267-73.
- Lavriv DS, Neves PM, Ravasco P. Should omega-3 fatty acids be used for adjuvant treatment of cancer cachexia? Clin Nutr ESPEN. 2018 Jun;25:18-25.
- Ewaschuk JB, Almasud A, Mazurak VC. Role of n-3 fatty acids in muscle loss and myosteatosis. Applied Physiology, Nutrition, and Metabolism. 2014 Jun;39(6):654-62.
- Golkhalkhali B, Rajandram R et al. Strain-specific probiotic (microbial cell preparation) and omega-3 fatty acid in modulating quality of life and inflammatory markers in colorectal cancer patients: a randomized controlled trial. Asia-Pacific Journal of Clinical Oncology. 2018 Jun;14(3):179-191.
- Xie H, Chang YN. Omega-3 polyunsaturated fatty acids in the prevention of postoperative complications in colorectal cancer: a meta-analysis. OncoTargets and Therapy. 2016 Dec 9;9:7435-7443.
- Sorensen LS, Thorlacius-Ussing O et al. Randomized clinical trial of perioperative omega-3 fatty acid supplements in elective colorectal cancer surgery. British Journal of Surgery. 2014 Jan;101(2):33-42.
- Trabal J, Leyes P, Forga M, Maurel J. Potential usefulness of an EPA-enriched nutritional supplement on chemotherapy tolerability in cancer patients without overt malnutrition. Nutricion Hospitalaria. 2010 Sep-Oct;25(5):736-40.
- Read JA, Beale PJ et al. Nutrition intervention using an eicosapentaenoic acid (EPA)-containing supplement in patients with advanced colorectal cancer. Effects on nutritional and inflammatory status: a phase II trial. Supportive Care in Cancer. 2007 Mar;15(3):301-7.
- Pappalardo G, Almeida A, Ravasco P. Eicosapentaenoic acid in cancer improves body composition and modulates metabolism. Nutrition. 2015 Apr;31(4):549-55.
- Wang YH, Yao N et al. The efficacy and safety of probiotics for prevention of chemoradiotherapy-induced diarrhea in people with abdominal and pelvic cancer: a systematic review and meta-analysis. European Journal of Clinical Nutrition. 2016;70(11):1246–1253.
- Lee JY, Chu SH et al. Effects of 12 weeks of probiotic supplementation on quality of life in colorectal cancer survivors: a double-blind, randomized, placebo-controlled trial. Digestive and Liver Disease. 2014;46(12):1126–1132.
- Anderson SW, Bazzell AF, Dains JE. An integrative review on the effect of prebiotics, probiotics, and synbiotics on infection after colorectal cancer surgery. AORN Journal. 2018;107(2):237–248; Aisu N, Tanimura S et al. Impact of perioperative probiotic treatment for surgical site infections in patients with colorectal cancer. Experimental and Therapeutic Medicine. 2015;10(3):966–972.
- Abrams DI. The therapeutic effects of cannabis and cannabinoids: an update from the National Academies of Sciences, Engineering and Medicine report. European Journal of Internal Medicine. 2018 Mar;49:7-11.
- Bar-Lev Schleider L, Mechoulam R et al. Prospective analysis of safety and efficacy of medical cannabis in large unselected population of patients with cancer. European Journal of Internal Medicine. 2018 Mar;49:37-43.
- Häuser W, Welsch P, Klose P, Radbruch L, Fitzcharles MA. Efficacy, tolerability and safety of cannabis-based medicines for cancer pain: a systematic review with meta-analysis of randomised controlled trials. Der Schmerz. 2019 Oct;33(5):424-436.
- Milla P, Airoldi M et al. Administration of reduced glutathione in FOLFOX4 adjuvant treatment for colorectal cancer: effect on oxaliplatin pharmacokinetics, Pt-DNA adduct formation, and neurotoxicity. Anticancer Drugs. 2009 Jun;20(5):396-402.
- Fu X, Wu H et al. Efficacy of drug interventions for chemotherapy-induced chronic peripheral neurotoxicity: a network meta-analysis. Frontiers in Neurology. 2017 Jun 8;8:223.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Serefko A, Szopa A, Poleszak E. Magnesium and depression. Magnesium Research. 2016;29(3):112–119; Cuciureanu MD, Vink R. Magnesium and stress. In Magnesium in the Central Nervous System [Internet]. Adelaide (AU): University of Adelaide Press; 2011.
- Boyle NB, Lawton C, Dye L. The effects of magnesium supplementation on subjective anxiety and stress—a systematic review. Nutrients. 2017;9(5):429.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Bock PR, Hanisch J, Matthes H, Zänker KS. Targeting inflammation in cancer-related-fatigue: a rationale for mistletoe therapy as supportive care in colorectal cancer patients. Inflammation & Allergy Drug Targets. 2014;13(2):105-11.
- Friedel WE, Matthes H, Bock PR, Zänker KS. Systematic evaluation of the clinical effects of supportive mistletoe treatment within chemo- and/or radiotherapy protocols and long-term mistletoe application in nonmetastatic colorectal carcinoma: multicenter, controlled, observational cohort study. Journal of the Society for Integrative Oncology. 2009 Fall;7(4):137-45.
- Thronicke A, Oei SL, Merkle A, Matthes H, Schad F. Clinical safety of combined targeted and Viscum album L. therapy in oncological patients. Medicines (Basel). 2018 Sep 6;5(3). pii: E100.
- Alschuler LN, Gazella KA. The Definitive Guide to Cancer, 3rd Edition: An Integrative Approach to Prevention, Treatment, and Healing. Berkeley, California: Celestial Arts. 2010; Alschuler LN, Gazella KA. The Definitive Guide to Thriving after Cancer: A Five-Step Integrative Plan to Reduce the Risk of Recurrence and Build Lifelong Health. Berkeley, California: Ten Speed Press. 2013.
- McKinney N. Naturopathic Oncology, 3rd Edition. Victoria, BC, Canada: Liaison Press. 2016.
- Parmar G, Kaczor T. Textbook of Naturopathic Oncology: A Desktop Guide of Integrative Cancer Care. 1st edition. Canada: Medicatrix Holdings Ltd. 2020.
- Ali BH. Amelioration of oxaliplatin neurotoxicity by drugs in humans and experimental animals: a minireview of recent literature. Basic & Clinical Pharmacology & Toxicology. 2010;106(4):272–279; Lin PC, Lee MY et al. N-acetylcysteine has neuroprotective effects against oxaliplatin-based adjuvant chemotherapy in colon cancer patients: preliminary data. Supportive Care in Cancer. 2006;14(5):484–487.
- Schloss JM, Colosimo M, Airey C, Masci PP, Linnane AW, Vitetta L. Nutraceuticals and chemotherapy induced peripheral neuropathy (CIPN): a systematic review [published correction appears in Clinical Nutrition. 2015 Feb;34(1):167]. Clinical Nutrition. 2013;32(6):888–893; Albers JW, Chaudhry V, Cavaletti G, Donehower RC. Interventions for preventing neuropathy caused by cisplatin and related compounds. Cochrane Database of Systematic Reviews. 2014;(3):CD005228; Ali BH. Amelioration of oxaliplatin neurotoxicity by drugs in humans and experimental animals: a minireview of recent literature. Basic and Clinical Pharmacology and Toxicology. 2010;106(4):272–279.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Wang QZ, Chen XP, Huang JP, Jiang XW. [Effects of couplet medicines (Astragalus membranaceus and jiaozhen) on intestinal barrier in postoperative colorectal cancer patients] [Article in Chinese]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2015;35(11):1307–1312.
- Grothey A, Nikcevich DA et al. Intravenous calcium and magnesium for oxaliplatin-induced sensory neurotoxicity in adjuvant colon cancer: NCCTG N04C7. Journal of Clinical Oncology. 2011 Feb 1;29(4):421-7.
- Gamelin L, Boisdron-Celle M et al. Prevention of oxaliplatin-related neurotoxicity by calcium and magnesium infusions: a retrospective study of 161 patients receiving oxaliplatin combined with 5-Fluorouracil and leucovorin for advanced colorectal cancer. Clinical Cancer Research. 2004 Jun 15;10(12 Pt 1):4055-61.
- Wu Z, Ouyang J, He Z, Zhang S. Infusion of calcium and magnesium for oxaliplatin-induced sensory neurotoxicity in colorectal cancer: a systematic review and meta-analysis. European Journal of Cancer. 2012 Aug;48(12):1791-8.
- Huang S, Peng W et al. Kangai injection, a traditional Chinese medicine, improves efficacy and reduces toxicity of chemotherapy in advanced colorectal cancer patients: a systematic review and meta-analysis. Evidence-based Complementary and Alternative Medicine. 2019 Jul 15;2019:8423037.
- Yu R, Wu X, Jia L, Lou Y. Effect of Chinese herbal compound LC09 on patients with capecitabine-associated hand-foot syndrome: a randomized, double-blind, and parallel-controlled trial. Integrative Cancer Therapies. Jan-Dec 2020;19:1534735420928466.
- Mantovani G, Macciò A et al. Randomized phase III clinical trial of five different arms of treatment in 332 patients with cancer cachexia. Oncologist. 2010;15(2):200-11.
- Yang YF, Xu Y, Wu Y. [Clinical randomized double-blinded controlled study on Quxie Capsule in reducing post-operational relapse and metastasis of colorectal cancer]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2007;27(10):879–882.
- Lagoa R, Silva J, Rodrigues JR, Bishayee A. Advances in phytochemical delivery systems for improved anticancer activity. Biotechnology Advances. 2019 Apr 9. pii: S0734-9750(19)30063-1; Amalraj A, Pius A, Gopi S, Gopi S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—a review. Journal of Traditional and Complementary Medicine. 2016 Jun 15;7(2):205-233; Belcaro G, Hosoi M et al. A controlled study of a lecithinized delivery system of curcumin (Meriva®) to alleviate the adverse effects of cancer treatment. Phytotherapy Research. 2014 Mar;28(3):444-50.
- Amalraj A, Pius A, Gopi S, Gopi S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—a review. Journal of Traditional and Complementary Medicine. 2016 Jun 15;7(2):205-233.
- Panahi Y, Saadat A, Beiraghdar F, Sahebkar A. Adjuvant therapy with bioavailability-boosted curcuminoids suppresses systemic inflammation and improves quality of life in patients with solid tumors: a randomized double-blind placebo-controlled trial. Phytotherapy Research. 2014 Oct;28(10):1461-7.
- Yeung KS, Gubili J, Mao JJ. Herb-drug interactions in cancer care. Oncology (Williston Park). 2018 Oct 15;32(10):516-20; Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS Journal. 2013 Jan;15(1):195-218.
- Yeung KS, Gubili J, Mao JJ. Herb-drug interactions in cancer care. Oncology (Williston Park). 2018 Oct 15;32(10):516-20.
- Amalraj A, Pius A, Gopi S, Gopi S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—a review. Journal of Traditional and Complementary Medicine. 2016 Jun 15;7(2):205-233.
- Chen D, Zhao J, Cong W. Chinese herbal medicines facilitate the control of chemotherapy-induced side effects in colorectal cancer: progress and perspective. Frontiers in Pharmacology. 2018 Dec 7;9:1442.
- Mueller T, Voigt W. Fermented wheat germ extract—nutritional supplement or anticancer drug? Nutrition Journal. 2011 Sep 5;10:89; Yeend T, Robinson K, Lockwood C, McArthur A. The effectiveness of fermented wheat germ extract as an adjunct therapy in the treatment of cancer: a systematic review. JBI Library of Systematic Reviews. 2012;10(42 Suppl):1-12.
- Yeend T, Robinson K, Lockwood C, McArthur A. The effectiveness of fermented wheat germ extract as an adjunct therapy in the treatment of cancer: a systematic review. JBI Library of Systematic Reviews. 2012;10(42 Suppl):1-12.
- Yehia R, Saleh S, El Abhar H, Saad AS, Schaalan M. L-Carnosine protects against oxaliplatin-induced peripheral neuropathy in colorectal cancer patients: a perspective on targeting Nrf-2 and NF-κB pathways. Toxicology and Applied Pharmacology. 2019;365:41-50.
- Harvie M. Nutritional supplements and cancer: potential benefits and proven harms. American Society of Clinical Oncology Educational Book. 2014;e478-e486.
- Schloss J, Colosimo M. B vitamin complex and chemotherapy-induced peripheral neuropathy. Current Oncology Reports. 2017;19(12):76; Schloss JM, Colosimo M et al. A randomised, placebo-controlled trial assessing the efficacy of an oral B group vitamin in preventing the development of chemotherapy-induced peripheral neuropathy (CIPN). Supportive Care in Cancer. 2017;25(1):195–204; Rostock M, Jaroslawski K et al. Chemotherapy-induced peripheral neuropathy in cancer patients: a four-arm randomized trial on the effectiveness of electroacupuncture. Evidence-Based Complementary and Alternative Medicine. 2013;2013:349653; Schloss JM, Colosimo M, Airey C, Masci PP, Linnane AW, Vitetta L. Nutraceuticals and chemotherapy induced peripheral neuropathy (CIPN): a systematic review [published correction appears in Clinical Nutrition. 2015 Feb;34(1):167]. Clinical Nutrition. 2013;32(6):888–893; Kim PY, Johnson CE. Chemotherapy-induced peripheral neuropathy: a review of recent findings. Current Opinion in Anesthesiology. 2017;30(5):570–576.
- Zhang M, Han W, Hu S, Xu H. Methylcobalamin: a potential vitamin of pain killer. Neural Plasticity. 2013;2013:424651.
- Yeom CH, Jung GC, Song KJ. Changes of terminal cancer patients' health-related quality of life after high dose vitamin C administration. Journal of Korean Medical Science. 2007 Feb;22(1):7-11.
- Takahashi H, Mizuno Haruyoshi Yanagisawa A. High-dose intravenous vitamin C improves quality of life in cancer patients. Personalized Medicine Universe. 2012 Jul;1(1):49-53.
- El Halabi I, Bejjany R et al. Ascorbic acid in colon cancer: from the basic to the clinical applications. International Journal of Moleculal Sciences. 2018;19(9):2752.
- Harvie M. Nutritional supplements and cancer: potential benefits and proven harms. American Society of Clinical Oncology Educational Book. 2014;e478-e486.
- Harvie M. Nutritional supplements and cancer: potential benefits and proven harms. American Society of Clinical Oncology Educational Book. 2014;e478-e486.
- Horie T, Matsumoto H et al. Protective effect of aged garlic extract on the small intestinal damage of rats induced by methotrexate administration. Planta Medica. 1999 Aug;65(6):545-8; Horie T, Awazu S, Itakura Y, Fuwa T. Alleviation by garlic of antitumor drug-induced damage to the intestine. Journal of Nutrition. 2001 Mar;131(3s):1071S-4S.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Cheah KY, Howarth GS, Bastian SE. Grape seed extract dose-responsively decreases disease severity in a rat model of mucositis; concomitantly enhancing chemotherapeutic effectiveness in colon cancer cells. PLoS One. 2014 Jan 21;9(1):e85184.
- Zhong Z, Wheeler MD et al. L-glycine: a novel antiinflammatory, immunomodulatory, and cytoprotective agent. Current Opinions in Clinical Nutrition and Metabolic Care. 2003 Mar;6(2):229-40; Mikalauskas S, Mikalauskiene L et al. Dietary glycine protects from chemotherapy-induced hepatotoxicity. Amino Acids. 2011 Apr;40(4):1139-50.
- Elliott WJ. Timing treatment to the rhythm of disease: a short course in chronotherapeutics. Postgraduate Medicine. 2001 Aug;110(2):119-22, 125-6, 129; Lévi F, Zidani R, Misset JL. Randomised multicentre trial of chronotherapy with oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer. International Organization for Cancer Chronotherapy. Lancet. 1997 Sep 6;350(9079):681-6.
- Lévi F, Zidani R, Misset JL. Randomised multicentre trial of chronotherapy with oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer. International Organization for Cancer Chronotherapy. Lancet. 1997 Sep 6;350(9079):681-6.
- Lévi FA, Zidani R et al. Chronomodulated versus fixed-infusion-rate delivery of ambulatory chemotherapy with oxaliplatin, fluorouracil, and folinic acid (leucovorin) in patients with colorectal cancer metastases: a randomized multi-institutional trial. Journal of the National Cancer Institute. 1994 Nov 2;86(21):1608-17.
- Baker D. Application of chronotherapy to the treatment of cancer: can changing the timing of drug administration influence efficacy and toxicity? Advances in Pharmacy. 2004 Jul;2(3):222-228.
- Mormont MC, Waterhouse J et al. Marked 24-h rest/activity rhythms are associated with better quality of life, better response, and longer survival in patients with metastatic colorectal cancer and good performance status. Clinical Cancer Research. 2000;6(8):3038–3045.
- Lévi F, Focan C et al. Implications of circadian clocks for the rhythmic delivery of cancer therapeutics. Advanced Drug Delivery Reviews. 2007;59(9-10):1015–1035.
- Boughattas NA, Lévi F et al. Stable circadian mechanisms of toxicity of two platinum analogs (cisplatin and carboplatin) despite repeated dosages in mice. Journal of Pharmacology and Experimental Therapeutics. 1990 Nov;255(2):672-9.
- El-Fatatry BM, Ibrahim OM, Hussien FZ, Mostafa TM. Role of metformin in oxaliplatin-induced peripheral neuropathy in patients with stage III colorectal cancer: randomized, controlled study. International Journal of Colorectal Disease. 2018 Dec;33(12):1675-1683.
- Patterson S. Metformin may have broad utility in cancer. MD Anderson Cancer Center. November-December 2014. Viewed January 19, 2018.
- Zhou W, Kavelaars A, Heijnen CJ. Metformin prevents cisplatin-induced cognitive impairment and brain damage in mice. PLoS ONE. 2016;11(3):e0151890.
- Elwood PC, Morgan G et al. Aspirin in the treatment of cancer: reductions in metastatic spread and in mortality: a systematic review and meta-analyses of published studies. PLoS One. 2016 Apr 20;11(4):e0152402.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Choi J, Lee EJ, Yang SH, Im YR, Seong J. A prospective Phase II study for the efficacy of radiotherapy in combination with zoledronic acid in treating painful bone metastases from gastrointestinal cancers. Journal of Radiation Research. 2019;60(2):242–248.
- Pantziarka P, Bouche G, Meheus L, Sukhatme V, Sukhatme VP. Repurposing drugs in oncology (ReDO)—cimetidine as an anti-cancer agent. Ecancermedicalscience. 2014;8:485.
- Pantziarka P, Bouche G, Meheus L, Sukhatme V, Sukhatme VP. Repurposing drugs in oncology (ReDO)—cimetidine as an anti-cancer agent. Ecancermedicalscience. 2014;8:485.
- Qu B, Qu H. The influence of statins on risk and patient survival in colorectal cancer. Journal of Clinical Gastroenterology. 2019;53(9):699–701.
- Block KI, Block PB, Gyllenhaal C. Integrative treatment for colorectal cancer: a comprehensive approach. Journal of Alternative and Complementary Medicine. 2018;24(9-10):890–901.
- Safdie FM, Dorff T, Quinn D, et al. Fasting and cancer treatment in humans: a case series report. Aging (Albany NY). 2009;1(12):988–1007.
- Dorff TB, Groshen S et al. Safety and feasibility of fasting in combination with platinum-based chemotherapy. BMC Cancer. 2016;16:360.
- Lévesque S, Pol JG, Ferrere G, Galluzzi L, Zitvogel L, Kroemer G. Trial watch: dietary interventions for cancer therapy. Oncoimmunology. 2019;8(7):1591878.
- Dorff TB, Groshen S et al. Safety and feasibility of fasting in combination with platinum-based chemotherapy. BMC Cancer. 2016;16:360.
- Jongbloed F, Huisman SA, van Steeg H, et al. The transcriptomic response to irinotecan in colon carcinoma bearing mice preconditioned by fasting. Oncotarget. 2019;10(22):2224–2234.
- Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Research Reviews. 2017;39:46–58; Nencioni A, Caffa I, Cortellino S, Longo VD. Fasting and cancer: molecular mechanisms and clinical application. Nature Reviews. Cancer 2018;18(11):707–719.
- Cheng CW, Adams Gregor B et al. Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosuppression. Cell Stem Cell. 2014;14:810–823.
- Block KI, Block PB, Gyllenhaal C. Integrative treatment for colorectal cancer: a comprehensive approach. Journal of Alternative and Complementary Medicine. 2018;24(9-10):890–901.
- Tusek DL, Church JM, Strong SA, Grass JA, Fazio VW. Guided imagery: a significant advance in the care of patients undergoing elective colorectal surgery. Diseases of the Colon and Rectum. 1997 Feb;40(2):172-8.
- Derksen TM, Bours MJ, Mols F, Weijenberg MP. Lifestyle-related factors in the self-management of chemotherapy-induced peripheral neuropathy in colorectal cancer: a systematic review. Evidence-Based Complementary and Alternative Medicine. 2017;2017:7916031.
- Chien A, Yang CC et al. Ultrasound acupuncture for oxaliplatin-induced peripheral neuropathy in patients with colorectal cancer: a pilot study. PM&R. 2020;10.1002/pmrj.12361.
- Gu XY, Gao ZQ, Zhang ZJ, Huang ZM, Xie XH. [Influence of warming needling technique on gastrointestinal reaction after hyperthermic intrape-ritoneal chemotherapy in patients with postoperation of colon cancer] [Article in Chinese]. Zhen Ci Yan Jiu. 2020;45(4):315-319.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Care. New York: Bantam Dell. 2009; Ning Y, Wang L, Giovannucci EL. A quantitative analysis of body mass index and colorectal cancer: findings from 56 observational studies. Obesity Reviews. 2010 Jan;11(1):19-30; Bardou M, Barkun AN, Martel M. Obesity and colorectal cancer. Gut. 2013 Jun;62(6):933-47; Shaukat A, Dostal A, Menk J, Church TR. BMI is a risk factor for colorectal cancer mortality. Digestive Diseases and Sciences. 2017 Sep;62(9):2511-2517; Lee J, Jeon JY, Meyerhardt JA. Diet and lifestyle in survivors of colorectal cancer. Hematology/Oncology Clinics of North America. 2015 Feb;29(1):1-27; National Cancer Institute. Colorectal Cancer Prevention (PDQ®)–Patient Version. March 15, 2019. Viewed May 14, 2019; Thanikachalam K, Khan G. Colorectal cancer and nutrition. Nutrients. 2019 Jan 14;11(1). pii: E164; Brand MP, Peeters PH, van Gils CH, Elias SG. Pre-adult famine exposure and subsequent colorectal cancer risk in women. International Journal of Epidemiology. 2017 Apr 1;46(2):612-621; Qu B, Qu H. The influence of statins on risk and patient survival in colorectal cancer. Journal of Clinical Gastroenterology. 2019;53(9):699–701; Ali Khan U, Fallah M et al. Personal history of diabetes as important as family history of colorectal cancer for risk of colorectal cancer: a nationwide cohort study. American Journal of Gastroenterology. 2020;10.14309/ajg.0000000000000669.
- Mayo Clinic Staff. Metabolic syndrome. Mayo Clinic. Viewed February 22, 2021.
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Diet, nutrition, physical activity and colorectal cancer. Viewed May 14, 2019; American Cancer Society: Can Colorectal Cancer Be Prevented? May 30, 2018. Viewed May 14, 2019; National Cancer Institute: Colorectal Cancer Prevention (PDQ®)–Patient Version. March 15, 2019. Viewed May 14, 2019; American Institute for Cancer Research. Cancer Prevention Recommendations. Viewed May 14, 2019; Wang X, Ji A et al. A meta-analysis including dose-response relationship between night shift work and the risk of colorectal cancer. Oncotarget. 2015 Sep 22;6(28):25046-60; Parent ME, El-Zein M, Rousseau M-C, Pintos J, Siemiatycki J. Night work and the risk of cancer among men. American Journal of Epidemiology. 2012 Nov 1;176(9):751-9; Hrushesky WJ, Lannin D, Haus E. Evidence for an ontogenetic basis for circadian coordination of cancer cell proliferation. Journal of the National Cancer Institute. 1998 Oct 7;90(19):1480-4; Durko L, Malecka-Panas E et al. Lifestyle modifications and colorectal cancer. Current Colorectal Cancer Reports. 2014;10:45-54; Garcia H, Song M. Early-life obesity and adulthood colorectal cancer risk: a meta-analysis. Revista Panamericana de Salud Publica. 2019 Jan 4;43:e3; Thanikachalam K, Khan G. Colorectal cancer and nutrition. Nutrients. 2019 Jan 14;11(1). pii: E164; Vainio H, Kaaks R, Bianchini F. Weight control and physical activity in cancer prevention: international evaluation of the evidence. European Journal of Cancer Prevention. 2002 Aug;11 Suppl 2:S94-100; Bailie L, Loughrey MB, Coleman HG. Lifestyle risk factors for serrated colorectal polyps: a systematic review and meta-analysis. Gastroenterology. 2017 Jan;152(1):92-104; Song M, Chan AT. Environmental factors, gut microbiota, and colorectal cancer prevention. Clinical Gastroenterology and Hepatology. 2019 Jan;17(2):275-289; Qu B, Qu H. The influence of statins on risk and patient survival in colorectal cancer. Journal of Clinical Gastroenterology. 2019;53(9):699–701; Dolejs SC, Gayed B, Fajardo A. Prevention of colorectal neoplasia. Clinics in Colon and Rectal Surgery. 2016;29(4):353-362; Derry MM, Raina K, Agarwal C, Agarwal R. Identifying molecular targets of lifestyle modifications in colon cancer prevention. Frontiers in Oncology. 2013 May 14;3:119; Kuipers EJ, Grady WM et al. Colorectal cancer. Nature Reviews. Disease Primers. 2015;1:15065; Chapelle N, Martel M, Toes-Zoutendijk E, Barkun AN, Bardou M. Recent advances in clinical practice: colorectal cancer chemoprevention in the average-risk population. Gut. 2020 Sep 28:gutjnl-2020-320990.
- Arthur RS, Dannenberg AJ, Kim M, Rohan TE. The association of body fat composition with risk of breast, endometrial, ovarian and colorectal cancers among normal weight participants in the UK Biobank. British Journal of Cancer. 2021 Mar 15.
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Diet, nutrition, physical activity and colorectal cancer. Viewed October 5, 2020.
- Keum N, Aune D, Greenwood DC, Ju W, Giovannucci EL. Calcium intake and colorectal cancer risk: dose-response meta-analysis of prospective observational studies. International Journal of Cancer. 2014 Oct 15;135(8):1940-8. Dolejs SC, Gayed B, Fajardo A. Prevention of colorectal neoplasia. Clinics in Colon and Rectal Surgery. 2016;29(4):353-362.
- Thanikachalam K, Khan G. Colorectal cancer and nutrition. Nutrients. 2019 Jan 14;11(1). pii: E164; Cauley JA, Chlebowski RT et al. Calcium plus vitamin D supplementation and health outcomes five years after active intervention ended: the Women's Health Initiative. Journal of Women’s Health (Larchmt). 2013 Nov;22(11):915-29; Katona BW, Weiss JM. Chemoprevention of colorectal cancer. Gastroenterology. 2020;158(2):368–388.
- Chen GC, Pang Z, Liu QF. Magnesium intake and risk of colorectal cancer: a meta-analysis of prospective studies. European Journal of Clinical Nutrition. 2012 Nov;66(11):1182-6; Chapelle N, Martel M, Toes-Zoutendijk E, Barkun AN, Bardou M. Recent advances in clinical practice: colorectal cancer chemoprevention in the average-risk population. Gut. 2020 Dec;69(12):2244-2255.
- Ohwada S, Ikeya T et al. Adjuvant immunochemotherapy with oral Tegafur/Uracil plus PSK in patients with stage II or III colorectal cancer: a randomised controlled study. British Journal of Cancer. 2004 Mar 8;90(5):1003-10.
- Oka S, Tanaka S et al. A water-soluble extract from culture medium of Ganoderma lucidum mycelia suppresses the development of colorectal adenomas. Hiroshima Journal of Medical Sciences. 59 (1): 1-6, 2010.
- Xie JT, Wang CZ et al. Ganoderma lucidum extract inhibits proliferation of SW 480 human colorectal cancer cells. Experimental Oncology. 2006 Mar;28(1):25-9.
- Ben S, Du M et al. Vitamin B2 intake reduces the risk for colorectal cancer: a dose-response analysis. European Journal of Nutrition. 2019;58(4):1591–1602.
- Thanikachalam K, Khan G. Colorectal cancer and nutrition. Nutrients. 2019 Jan 14;11(1). pii: E164; Larsson SC, Orsini N, Wolk A. Vitamin B6 and risk of colorectal cancer: a meta-analysis of prospective studies. JAMA. 2010 Mar 17;303(11):1077-83.
- Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS Journal . 2013 Jan;15(1):195-218; Carroll RE, Benya RV et al. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prevention Research (Philadelphia, Pennsylvania). 2011 Mar;4(3):354-64; Fadus MC, Lau C, Bikhchandani J, Lynch HT. Curcumin: an age-old anti-inflammatory and anti-neoplastic agent. Journal of Traditional and Complementary Medicine. 2016 Sep 9;7(3):339-346; Cruz-Correa M, Shoskes DA, Sanchez P, Zhao R, Hylind LM, Wexner SD, Giardiello FM. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clinical Gastroenterology and Hepatology. 2006 Aug;4(8):1035-8. .
- Gulbake A, Jain A, Jain A, Jain A, Jain SK. Insight to drug delivery aspects for colorectal cancer. World Journal of Gastroenterology. 2016 Jan 14;22(2):582-99.
- Alschuler LN, Gazella KA. The Definitive Guide to Cancer, 3rd Edition: An Integrative Approach to Prevention, Treatment, and Healing. Berkeley, California: Celestial Arts. 2010; Alschuler LN, Gazella KA. The Definitive Guide to Thriving after Cancer: A Five-Step Integrative Plan to Reduce the Risk of Recurrence and Build Lifelong Health. Berkeley, California: Ten Speed Press. 2013.
- Bar-Sela G, Epelbaum R, Schaffer M. Curcumin as an anti-cancer agent: review of the gap between basic and clinical applications. Current Medicinal Chemistry. 2010;17(3):190-7.
- Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS Journal. 2013 Jan;15(1):195-218; Carroll RE, Benya RV et al. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prevention Research (Philadelphia, Pennsylvania). 2011 Mar;4(3):354-64.
- Amalraj A, Pius A, Gopi S, Gopi S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—a review. Journal of Traditional and Complementary Medicine. 2016 Jun 15;7(2):205-233.
- Núñez-Sánchez MA, González-Sarrías A et al. Dietary phenolics against colorectal cancer—from promising preclinical results to poor translation into clinical trials: pitfalls and future needs. Molecular Nutrition & Food Research. 2015 Jul;59(7):1274-91.
- Fadus MC, Lau C, Bikhchandani J, Lynch HT. Curcumin: an age-old anti-inflammatory and anti-neoplastic agent. Journal of Traditional and Complementary Medicine. 2016 Sep 9;7(3):339-346; Boral D, Nie D. Cancer stem cells and niche mircoenvironments. Frontiers in Bioscience (Elite Edition). 2012 Jun 1;4:2502-14.
- Hou TY, Davidson LA et al. Nutrient-gene interaction in colon cancer, from the membrane to cellular physiology. Annual Review of Nutrition. 2016 Jul 17;36:543-70.
- Alschuler LN, Gazella KA. The Definitive Guide to Cancer, 3rd Edition: An Integrative Approach to Prevention, Treatment, and Healing. Berkeley, California: Celestial Arts. 2010; Alschuler LN, Gazella KA. The Definitive Guide to Thriving after Cancer: A Five-Step Integrative Plan to Reduce the Risk of Recurrence and Build Lifelong Health. Berkeley, California: Ten Speed Press. 2013.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- McKinney N. Naturopathic Oncology, 3rd Edition. Victoria, BC, Canada: Liaison Press. 2016.
- Liu C, Li P et al. Advances in the antagonism of epigallocatechin-3-gallate in the treatment of digestive tract tumors. Molecules. 2019 May 3;24(9). pii: E1726; Kumar N, Shibata D, Helm J, Coppola D, Malafa M. Green tea polyphenols in the prevention of colon cancer. Frontiers in Bioscience. 2007 Jan 1;12:2309-15; Liu C, Li P et al. Advances in the antagonism of epigallocatechin-3-gallate in the treatment of digestive tract tumors. Molecules. 2019 May 3;24(9). pii: E1726.
- Shimizu M, Adachi S, Masuda M, Kozawa O, Moriwaki H. Cancer chemoprevention with green tea catechins by targeting receptor tyrosine kinases. Molecular Nutrition & Food Research. 2011 Jun;55(6):832-43.
- Shimizu M, Fukutomi Y et al. Green tea extracts for the prevention of metachronous colorectal adenomas: a pilot study. Cancer Epidemiology, Biomarkers & Prevention. 2008 Nov;17(11):3020-5; Shin CM, Lee DH et al. Green tea extracts for the prevention of metachronous colorectal polyps among patients who underwent endoscopic removal of colorectal adenomas: a randomized clinical trial. Clinical Nutrition. 2018 Apr;37(2):452-458.
- Chen J, Huang XF. The signal pathways in azoxymethane-induced colon cancer and preventive implications. Cancer Biology & Therapy. 2009 Jul;8(14):1313-7.
- Fajardo AM, Piazza GA. Chemoprevention in gastrointestinal physiology and disease. Anti-inflammatory approaches for colorectal cancer chemoprevention. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2015 Jul 15;309(2):G59-70; Ullah MF, Bhat SH et al. Pharmacological intervention through dietary nutraceuticals in gastrointestinal neoplasia. Critical Reviews in Food Science and Nutrition. 2016 Jul 3;56(9):1501-18.
- Ullah MF, Bhat SH et al. Pharmacological intervention through dietary nutraceuticals in gastrointestinal neoplasia. Critical Reviews in Food Science and Nutrition. 2016 Jul 3;56(9):1501-18.
- Ullah MF, Bhat SH et al. Pharmacological intervention through dietary nutraceuticals in gastrointestinal neoplasia. Critical Reviews in Food Science and Nutrition. 2016 Jul 3;56(9):1501-18.
- Hu G, Zhang L, Rong Y, Ni X, Sun Y. Downstream carcinogenesis signaling pathways by green tea polyphenols: a translational perspective of chemoprevention and treatment for cancers. Current Drug Metabolism. 2014 Jan;15(1):14-22.
- Alschuler LN, Gazella KA. The Definitive Guide to Cancer, 3rd Edition: An Integrative Approach to Prevention, Treatment, and Healing. Berkeley, California: Celestial Arts. 2010; Alschuler LN, Gazella KA. The Definitive Guide to Thriving after Cancer: A Five-Step Integrative Plan to Reduce the Risk of Recurrence and Build Lifelong Health. Berkeley, California: Ten Speed Press. 2013.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Lemole G, Mehta P, McKee D. After Cancer Care: The Definitive Self-Care Guide to Getting and Staying Well for Patients with Cancer. New York, New York: Rodale, Inc. 2015.
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Diet, nutrition, physical activity and colorectal cancer. Viewed May 14, 2019.
- Zhang SM, Moore SC et al. Folate, vitamin B6, multivitamin supplements, and colorectal cancer risk in women. American Journal of Epidemiology. 2006;163(2):108–115.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Kim S, Sandler DP, Galanko J, Martin C, Sandler RS. Intake of polyunsaturated fatty acids and distal large bowel cancer risk in whites and African Americans. American Journal of Epidemiology. 2010 May 1;171(9):969-79; Satia JA, Littman A, Slatore CG, Galanko JA, White E.Associations of herbal and specialty supplements with lung and colorectal cancer risk in the VITamins and Lifestyle study. Cancer Epidemiology, Biomarkers & Prevention. 2009 May;18(5):1419-28.
- Kantor ED, Lampe JW, Peters U, Vaughan TL, White E. Long-chain omega-3 polyunsaturated fatty acid intake and risk of colorectal cancer. Nutrition and Cancer. 2014;66(4):716-27.
- Katona BW, Weiss JM. Chemoprevention of colorectal cancer. Gastroenterology. 2020;158(2):368–388.
- Zárate R, El Jaber-Vazdekis N, Tejera N, Pérez JA, Rodríguez C. Significance of long chain polyunsaturated fatty acids in human health. Clinical and Translational Medicine. 2017 Dec;6(1):25.
- Song M, Chan AT. Environmental factors, gut microbiota, and colorectal cancer prevention. Clinical Gastroenterology and Hepatology. 2019 Jan;17(2):275-289.
- Hull MA, Sprange K et al. Eicosapentaenoic acid and aspirin, alone and in combination, for the prevention of colorectal adenomas (seAFOod Polyp Prevention trial): a multicentre, randomised, double-blind, placebo-controlled, 2 × 2 factorial trial. Lancet. 2018 Dec 15;392(10164):2583-2594.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Lemole G, Mehta P, McKee D. After Cancer Care: The Definitive Self-Care Guide to Getting and Staying Well for Patients with Cancer. New York, New York: Rodale, Inc. 2015.
- McKinney N. Naturopathic Oncology, 3rd Edition. Victoria, BC, Canada: Liaison Press. 2016.
- Hendler R, Zhang Y. Probiotics in the treatment of colorectal cancer. Medicines (Basel). 2018 Sep 7;5(3):101.
- Lamichhane P, Maiolini M et al. Colorectal cancer and probiotics: Are bugs really drugs? Cancers (Basel). 2020 May 5;12(5):1162; Drago L. Probiotics and colon cancer. Microorganisms. 2019 Feb 28;7(3):66.
- Zheng X, Wu K et al. Yogurt consumption and risk of conventional and serrated precursors of colorectal cancer. Gut. 2019;gutjnl-2019-318374.
- Jacouton E, Chain F, Sokol H, Langella P, Bermúdez-Humarán LG. Probiotic strain Lactobacillus casei BL23 prevents colitis-associated colorectal cancer. Frontiers in Immunology. 2017 Nov 17;8:1553.
- Gao C, Ganesh BP et al. Gut microbe-mediated suppression of inflammation-associated colon carcinogenesis by luminal histamine production. American Journal of Pathology. 2017 Oct;187(10):2323-2336.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- McKinney N. Naturopathic Oncology, 3rd Edition. Victoria, BC, Canada: Liaison Press. 2016.
- Nguyen AV, Martinez M et al. Results of a phase I pilot clinical trial examining the effect of plant-derived resveratrol and grape powder on Wnt pathway target gene expression in colonic mucosa and colon cancer. Cancer Management and Research. 2009 Apr 3;1:25-37.
- Alschuler LN, Gazella KA. The Definitive Guide to Cancer, 3rd Edition: An Integrative Approach to Prevention, Treatment, and Healing. Berkeley, California: Celestial Arts. 2010; Alschuler LN, Gazella KA. The Definitive Guide to Thriving after Cancer: A Five-Step Integrative Plan to Reduce the Risk of Recurrence and Build Lifelong Health. Berkeley, California: Ten Speed Press. 2013.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Crosara Teixeira M, Braghiroli MI, Sabbaga J, Hoff PM. Primary prevention of colorectal cancer: myth or reality? World Journal of Gastroenterology. 2014 Nov 7;20(41):15060-9; Song M, Chan AT. Environmental factors, gut microbiota, and colorectal cancer prevention. Clinical Gastroenterology and Hepatology. 2019 Jan;17(2):275-289; World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Diet, nutrition, physical activity and colorectal cancer. Viewed May 14, 2019; Thanikachalam K, Khan G. Colorectal cancer and nutrition. Nutrients. 2019 Jan 14;11(1). pii: E164; Shang M, Sun J. Vitamin D/VDR, probiotics, and gastrointestinal diseases. Current Medicinal Chemistry. 2017;24(9):876-887; Feskanich D, Ma J et al. Plasma vitamin D metabolites and risk of colorectal cancer in women. Cancer Epidemiology, Biomarkers & Prevention. 2004 Sep;13(9):1502-8; Lee JE, Li H et al. Circulating levels of vitamin D and colon and rectal cancer: the Physicians' Health Study and a meta-analysis of prospective studies. Cancer Prevention Research (Philadelphia). 2011 May;4(5):735-43; McCullough ML, Zoltick ES et al. Circulating vitamin D and colorectal cancer risk: an international pooling project of 17 cohorts. JNCI: Journal of the National Cancer Institute. 2019 Feb 1;111(2):158-169.
- Krstic MN, Mijac DD, Popovic DD, Pavlovic Markovic A, Milosavljević T. General aspects of primary cancer prevention. Digestive Diseases. 2019;37(5):406–415.
- Grau MV, Baron JA et al. Vitamin D, calcium supplementation, and colorectal adenomas: results of a randomized trial. JNCI: Journal of the National Cancer Institute. 2003;95(23):1765-1771.
- Chapelle N, Martel M, Toes-Zoutendijk E, Barkun AN, Bardou M. Recent advances in clinical practice: colorectal cancer chemoprevention in the average-risk population. Gut. 2020 Sep 28:gutjnl-2020-320990; Touvier M, Chan DS et alT. Meta-analyses of vitamin D intake, 25-hydroxyvitamin D status, vitamin D receptor polymorphisms, and colorectal cancer risk. Cancer Epidemiology, Biomarkers & Prevention. 2011 May;20(5):1003-16.
- Krstic MN, Mijac DD, Popovic DD, Pavlovic Markovic A, Milosavljević T. General aspects of primary cancer prevention. Digestive Diseases. 2019;37(5):406–415.
- Katona BW, Weiss JM. Chemoprevention of colorectal cancer. Gastroenterology. 2020;158(2):368–388.
- Ding EL, Mehta S, Fawzi WW, Giovannucci EL. Interaction of estrogen therapy with calcium and vitamin D supplementation on colorectal cancer risk: reanalysis of Women's Health Initiative randomized trial. International Journal of Cancer. 2008 Apr 15;122(8):1690-4.
- Beaty MM, Lee EY, Glauert HP. Influence of dietary calcium and vitamin D on colon epithelial cell proliferation and 1,2-dimethylhydrazine-induced colon carcinogenesis in rats fed high fat diets. Journal of Nutrition. 1993;123(1):144-152.
- Katona BW, Weiss JM. Chemoprevention of colorectal cancer. Gastroenterology. 2020;158(2):368–388.
- Alschuler LN, Gazella KA. The Definitive Guide to Cancer, 3rd Edition: An Integrative Approach to Prevention, Treatment, and Healing. Berkeley, California: Celestial Arts. 2010; Alschuler LN, Gazella KA. The Definitive Guide to Thriving after Cancer: A Five-Step Integrative Plan to Reduce the Risk of Recurrence and Build Lifelong Health. Berkeley, California: Ten Speed Press. 2013.
- Dong Y, Liu Y et al. Link between risk of colorectal cancer and serum vitamin E levels: a meta-analysis of case-control studies. Medicine (Baltimore). 2017 Jul;96(27):e7470.
- Ju J, Picinich SC, et al. Cancer-preventive activities of tocopherols and tocotrienols. Carcinogenesis. 2010;31(4):533-542; Yang CS, Suh N, Kong AN. Does vitamin E prevent or promote cancer? Cancer Prevention Research (Philadelphia). 2012;5(5):701-705.
- Campbell S, Stone W, Whaley S, Krishnan K. Development of gamma (gamma)-tocopherol as a colorectal cancer chemopreventive agent. Critical Reviews in Oncology/Hematology. 2003;47(3):249-259.
- Stone WL, Krishnan K, Campbell SE, Palau VE. The role of antioxidants and pro-oxidants in colon cancer. World Journal of Gastrointestinal Oncology. 2014 Mar 15;6(3):55-66.
- Lippman SM, Klein EA et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2009 Jan 7;301(1):39-51.
- Papaioannou D, Cooper KL et al. Antioxidants in the chemoprevention of colorectal cancer and colorectal adenomas in the general population: a systematic review and meta-analysis. Colorectal Disease. 2011 Oct;13(10):1085-99; Arain MA, Abdul Qadeer A. Systematic review on "vitamin E and prevention of colorectal cancer". Pakistan Journal of Pharmaceutical Sciences. 2010 Apr;23(2):125-30; Harvie M. Nutritional supplements and cancer: potential benefits and proven harms. American Society of Clinical Oncology Educational Book. 2014;e478-e486.
- Lance P, Alberts DS et al. Colorectal adenomas in participants of the SELECT randomized trial of selenium and vitamin E for prostate cancer prevention. Cancer Prevention Research (Philadelphia). 2017 Jan;10(1):45-54.
- Lemole G, Mehta P, McKee D. After Cancer Care: The Definitive Self-Care Guide to Getting and Staying Well for Patients with Cancer. New York, New York: Rodale, Inc. 2015.
- McKinney N. Naturopathic Oncology, 3rd Edition. Victoria, BC, Canada: Liaison Press. 2016.
- Boros LG, Nichelatti M, Shoenfeld Y. Fermented wheat germ extract (Avemar) in the treatment of cancer and autoimmune diseases. Annals of the New York Academy of Sciences. 2005 Jun;1051:529-42.
- Marshall JR. Prevention of colorectal cancer: diet, chemoprevention, and lifestyle. Gastroenterology Clinics of North America. 2008;37(1):73-vi
- Duffield-Lillico AJ, Reid ME et al. Baseline characteristics and the effect of selenium supplementation on cancer incidence in a randomized clinical trial: a summary report of the Nutritional Prevention of Cancer Trial. Cancer Epidemiology, Biomarkers & Prevention. 2002;11(7):630-639.
- Dong J, Liang W et al. Saponins regulate intestinal inflammation in colon cancer and IBD. Pharmacological Research. 2019;144:66–72.
- Reddivari L, Charepalli V et al. Grape compounds suppress colon cancer stem cells in vitro and in a rodent model of colon carcinogenesis. BMC Complementary and Alternative Medicine. 2016 Aug 9;16:278.
- Zick SM, Turgeon DK et al. Phase II study of the effects of ginger root extract on eicosanoids in colon mucosa in people at normal risk for colorectal cancer. Cancer Prevention Research (Philadelphia). 2011 Nov;4(11):1929-37.
- de Lima RMT, Dos Reis AC et al. Protective and therapeutic potential of ginger (Zingiber officinale) extract and [6]-gingerol in cancer: a comprehensive review. Phytotherapy Research. 2018 Oct;32(10):1885-1907; Núñez-Sánchez MA, González-Sarrías A et al. Dietary phenolics against colorectal cancer—from promising preclinical results to poor translation into clinical trials: pitfalls and future needs. Molecular Nutrition and Food Research. 2015 Jul;59(7):1274-91.
- Zhang M, Viennois E et al. Edible ginger-derived nanoparticles: a novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials. 2016 Sep;101:321-40.
- Derry MM, Raina K et al. Grape seed extract efficacy against azoxymethane-induced colon tumorigenesis in A/J mice: interlinking miRNA with cytokine signaling and inflammation. Cancer Prevention Research (Philadelphia). 2013 Jul;6(7):625-33; Derry MM, Raina K, Agarwal C, Agarwal R. Identifying molecular targets of lifestyle modifications in colon cancer prevention. Frontiers in Oncology. 2013 May 14;3:119; Velmurugan B, Singh RP, Agarwal R, Agarwal C. Dietary-feeding of grape seed extract prevents azoxymethane-induced colonic aberrant crypt foci formation in fischer 344 rats. Mol Carcinog. 2010 Jul;49(7):641-52; Velmurugan B, Singh RP, Kaul N, Agarwal R, Agarwal C. Dietary feeding of grape seed extract prevents intestinal tumorigenesis in APCmin/+ mice. Neoplasia. 2010 Jan;12(1):95-102.
- Velmurugan B, Singh RP, Agarwal R, Agarwal C. Dietary-feeding of grape seed extract prevents azoxymethane-induced colonic aberrant crypt foci formation in fischer 344 rats. Molecular Carcinogenesis. 2010 Jul;49(7):641-52.
- Velmurugan B, Singh RP, Kaul N, Agarwal R, Agarwal C. Dietary feeding of grape seed extract prevents intestinal tumorigenesis in APCmin/+ mice. Neoplasia. 2010 Jan;12(1):95-102.
- Satia JA, Littman A, Slatore CG, Galanko JA, White E.Associations of herbal and specialty supplements with lung and colorectal cancer risk in the VITamins and Lifestyle study. Cancer Epidemiology, Biomarkers & Prevention. 2009 May;18(5):1419-28; Zhu B, Zou L, Qi L, Zhong R, Miao X. Allium vegetables and garlic supplements do not reduce risk of colorectal cancer, based on meta-analysis of prospective studies. Clinical Gastroenterology and Hepatology. 2014 Dec;12(12):1991-2001.e1-4; quiz e121.
- Harvie M. Nutritional supplements and cancer: potential benefits and proven harms. American Society of Clinical Oncology Educational Book. 2014;e478-e486.
- Katona BW, Weiss JM. Chemoprevention of colorectal cancer. Gastroenterology. 2020;158(2):368–388; Dolejs SC, Gayed B, Fajardo A. Prevention of colorectal neoplasia. Clinics in Colon and Rectal Surgery. 2016;29(4):353-362.
- Bibbins-Domingo K; US Preventive Services Task Force. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: US Preventive Services Task Force recommendation statement. Annals of Internal Medicine. 2016 Jun 21;164(12):836-45.
- Bosetti C, Santucci C, Gallus S, Martinetti M, La Vecchia C. Aspirin and the risk of colorectal and other digestive tract cancers: an updated meta-analysis through 2019. Annals of Oncology. 2020;S0923-7534(20)36073-7; Bosetti C, Rosato V, Gallus S, Cuzick J, La Vecchia C. Aspirin and cancer risk: a quantitative review to 2011. Annals of Oncology. 2012 Jun;23(6):1403-15; Ye X, Fu J, Yang Y, Chen S. Dose-risk and duration-risk relationships between aspirin and colorectal cancer: a meta-analysis of published cohort studies. PLoS One. 2013;8(2):e57578; Yang T, Li X et al. International Journal of Cancer. Gene-environment interactions and colorectal cancer risk: an umbrella review of systematic reviews and meta-analyses of observational studies. 2019 Nov 1;145(9):2315-2329. doi: 10.1002/ijc.32057; Mohammed A, Yarla NS, Madka V, Rao CV. Clinically relevant anti-inflammatory agents for chemoprevention of colorectal cancer: new perspectives. International Journal of Molecular Sciences. 2018 Aug 8;19(8). pii: E2332; Chen J, Stark LA. Aspirin prevention of colorectal cancer: focus on NF-κB signalling and the nucleolus. Biomedicines. 2017 Jul 18;5(3). pii: E43; Bailie L, Loughrey MB, Coleman HG. Lifestyle risk factors for serrated colorectal polyps: a systematic review and meta-analysis. Gastroenterology. 2017 Jan;152(1):92-104; Piazuelo E, Lanas A. NSAIDS and gastrointestinal cancer. Prostaglandins & Other Lipid Mediators. 2015 Jul;120:91-6; Thanikachalam K, Khan G. Colorectal cancer and nutrition. Nutrients. 2019 Jan 14;11(1). pii: E164; Dolejs SC, Gayed B, Fajardo A. Prevention of colorectal neoplasia. Clinics in Colon and Rectal Surgery. 2016;29(4):353-362; Wakeman C, Keenan J et al. Chemoprevention of colorectal neoplasia. ANZ Journal of Surgery. 2017 Dec;87(12):E228-E232; Pan P, Huang YW et al. Could aspirin and diets high in fiber act synergistically to reduce the risk of colon cancer in humans? International Journal of Molecular Sciences. 2018 Jan 6;19(1). pii: E166; Chapelle N, Martel M, Toes-Zoutendijk E, Barkun AN, Bardou M. Recent advances in clinical practice: colorectal cancer chemoprevention in the average-risk population. Gut. 2020 Sep 28:gutjnl-2020-320990.
- Burn J, Sheth H et al; CAPP2 Investigators. Cancer prevention with aspirin in hereditary colorectal cancer (Lynch syndrome), 10-year follow-up and registry-based 20-year data in the CAPP2 study: a double-blind, randomised, placebo-controlled trial. Lancet. 2020 Jun 13;395(10240):1855-1863.
- Frouws MA, Reimers MS et al. The influence of BRAF and KRAS mutation status on the association between aspirin use and survival after colon cancer diagnosis.PLoS One. 2017 Jan 26;12(1):e0170775; Krstic MN, Mijac DD, Popovic DD, Pavlovic Markovic A, Milosavljević T. General aspects of primary cancer prevention. Digestive Diseases. 2019;37(5):406-415; Baron JA, Cole BF et al. A randomized trial of aspirin to prevent colorectal adenomas. New England Journal of Medicine. 2003 Mar 6;348(10):891-9; Sandler RS, Halabi S et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. New England Journal of Medicine. 2003 Mar 6;348(10):883-90.
- Cooper K, Squires H et al. Chemoprevention of colorectal cancer: systematic review and economic evaluation. Health Technol Assess. 2010 Jun;14(32):1-206; Umezawa S, Higurashi T et al. Chemoprevention of colorectal cancer: past, present, and future. Cancer Science. 2019 Oct;110(10):3018-3026; Serrano D, Bonanni B, Brown K. Therapeutic cancer prevention: achievements and ongoing challenges—a focus on breast and colorectal cancer. Molecular Oncology. 2019 Mar;13(3):579-590; Burn J, Gerdes AM et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet. 2011 Dec 17;378(9809):2081-7.
- Rothwell PM, Cook NR et al. Effects of aspirin on risks of vascular events and cancer according to bodyweight and dose: analysis of individual patient data from randomised trials. Lancet. 2018 Aug 4;392(10145):387-399.
- Loomans-Kropp HA, Pinsky P, Umar A. Evaluation of aspirin use with cancer incidence and survival among older adults in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. JAMA Network Open. 2021 Jan 4;4(1):e2032072.
- Guo CG, Ma W et al. Aspirin use and risk of colorectal cancer among older adults. JAMA Oncol. 2021 Jan 21.
- Mitrugno A, Sylman JL et al. Aspirin therapy reduces the ability of platelets to promote colon and pancreatic cancer cell proliferation: implications for the oncoprotein c-MYC. American Journal of Physiology. Cell Physiology. 2017 Feb 1;312(2):C176-C189; Patrignani P, Patrono C. Aspirin, platelet inhibition and cancer prevention. Platelets. 2018 Dec;29(8):779-785.
- Mitrugno A, Sylman JL et al. Aspirin therapy reduces the ability of platelets to promote colon and pancreatic cancer cell proliferation: implications for the oncoprotein c-MYC. American Journal of Physiology—Cell Physiology. 2017;312(2):C176–C189.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Chang R. Beyond the Magic Bullet: The Anti-Cancer Cocktail. New York: Square One Publishers. 2012.
- Vogtmann E, Corley DA et al. Oral bisphosphonates and colorectal cancer. Scientific Reports. 2017;7:44177; Ibáñez-Sanz G, Guinó E et al. Risk of colorectal cancer in users of bisphosphonates: analysis of population-based electronic health records. European Journal of Epidemiology. 2019;10.1007/s10654-019-00584-5; Eiken P, Vestergaard P. Oral bisphosphonates and colon cancer: an update. Therapeutic Advances in Musculoskeletal Disease. 2015;7(4):160–168; Ma J, Gao S, Ni X, et al. Exposure to bisphosphonates and risk of colorectal cancer. British Journal of Clinical Pharmacology. 2013;76(3):320–328; Thosani N, Thosani SN et al. Reduced risk of colorectal cancer with use of oral bisphosphonates: a systematic review and meta-analysis. Journal of Clinical Oncology. 2013;31(5):623–630; Singh S, Singh AG, Murad MH, Limburg PJ. Bisphosphonates are associated with reduced risk of colorectal cancer: a systematic review and meta-analysis. Clinical Gastroenterology and Hepatology. 2013;11(3):232–9.e1; Oh YH, Yoon C, Park SM. Bisphosphonate use and gastrointestinal tract cancer risk: meta-analysis of observational studies. World Journal of Gastroenterology. 2012;18(40):5779–5788; Dolejs SC, Gayed B, Fajardo A. Prevention of colorectal neoplasia. Clinics in Colon and Rectal Surgery. 2016;29(4):353-362.
- Song M, Chan AT. Environmental factors, gut microbiota, and colorectal cancer prevention. Clinical Gastroenterology and Hepatology. 2019 Jan;17(2):275-289; Katona BW, Weiss JM. Chemoprevention of colorectal cancer. Gastroenterology. 2019 Sep 26. pii: S0016-5085(19)41364-4.
- Joo MK, Park JJ, Chun HJ. Additional benefits of routine drugs on gastrointestinal cancer: statins, metformin, and proton pump inhibitors. Digestive Diseases. 2018;36(1):1-14; Rokkas T, Portincasa P. Colon neoplasia in patients with type 2 diabetes on metformin: a meta-analysis. European Journal of Internal Medicine. 2016 Sep;33:60-6; Krstic MN, Mijac DD, Popovic DD, Pavlovic Markovic A, Milosavljević T. General aspects of primary cancer prevention. Digestive Diseases. 2019;37(5):406-415; Katona BW, Weiss JM. Chemoprevention of colorectal cancer. Gastroenterology. 2019 Sep 26. pii: S0016-5085(19)41364-4; Krstic MN, Mijac DD, Popovic DD, Pavlovic Markovic A, Milosavljević T. General aspects of primary cancer prevention. Digestive Diseases. 2019;37(5):406–415.
- Paleari L, Burhenne J et al. High accumulation of metformin in colonic tissue of subjects with diabetes or the metabolic syndrome. Gastroenterology. 2018 Apr;154(5):1543-1545; Umezawa S, Higurashi T et al. Chemoprevention of colorectal cancer: past, present, and future. Cancer Science. 2019 Oct;110(10):3018-3026; Higurashi T, Hosono K et al. Metformin for chemoprevention of metachronous colorectal adenoma or polyps in post-polypectomy patients without diabetes: a multicentre double-blind, placebo-controlled, randomised phase 3 trial. Lancet Oncology. 2016 Apr;17(4):475-483.
- Higurashi T, Nakajima A. Metformin and colorectal cancer. Frontiers in Endocrinology (Lausanne). 2018 Oct 23;9:622.
- La Vecchia C, Bosetti C. Metformin: are potential benefits on cancer risk extended to cancer survival? The Oncologist. 2013 Dec;18(12):1245-1247.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Piazuelo E, Lanas A. NSAIDs and gastrointestinal cancer. Prostaglandins & Other Lipid Mediators. 2015;120:91–96; Chapelle N, Martel M, Toes-Zoutendijk E, Barkun AN, Bardou M. Recent advances in clinical practice: colorectal cancer chemoprevention in the average-risk population. Gut. 2020 Sep 28:gutjnl-2020-320990.
- Tomić T, Domínguez-López S, Barrios-Rodríguez R. Non-aspirin non-steroidal anti-inflammatory drugs in prevention of colorectal cancer in people aged 40 or older: a systematic review and meta-analysis. Cancer Epidemiology. 2019;58:52–62.
- Wakeman C, Keenan J et al. Chemoprevention of colorectal neoplasia. ANZ Journal of Surgery. 2017 Dec;87(12):E228-E232; Arber N, Eagle CJ et al. Celecoxib for the prevention of colorectal adenomatous polyps. New England Journal of Medicine. 2006 Aug 31;355(9):885-95; Bertagnolli MM, Eagle CJ et al. Celecoxib for the prevention of sporadic colorectal adenomas. New England Journal of Medicine. 2006 Aug 31;355(9):873-84; Mohammed A, Yarla NS, Madka V, Rao CV. Clinically relevant anti-inflammatory agents for chemoprevention of colorectal cancer: new perspectives. International Journal of Molecular Sciences. 2018 Aug 8;19(8). pii: E2332; Dolejs SC, Gayed B, Fajardo A. Prevention of colorectal neoplasia. Clinics in Colon and Rectal Surgery. 2016;29(4):353-362.
- Tomić T, Domínguez-López S, Barrios-Rodríguez R. Non-aspirin non-steroidal anti-inflammatory drugs in prevention of colorectal cancer in people aged 40 or older: a systematic review and meta-analysis. Cancer Epidemiology. 2019;58:52–62.
- Steinbach G, Lynch PM et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. New England Journal of Medicine. 2000 Jun 29;342(26):1946-52.
- Giardiello FM, Hamilton SR et al. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. New England Journal of Medicine. 1993 May 6;328(18):1313-6.
- Tomić T, Domínguez-López S, Barrios-Rodríguez R. Non-aspirin non-steroidal anti-inflammatory drugs in prevention of colorectal cancer in people aged 40 or older: a systematic review and meta-analysis. Cancer Epidemiology. 2019;58:52–62.
- Dolejs SC, Gayed B, Fajardo A. Prevention of colorectal neoplasia. Clinics in Colon and Rectal Surgery. 2016;29(4):353-362.
- Tomić T, Domínguez-López S, Barrios-Rodríguez R. Non-aspirin non-steroidal anti-inflammatory drugs in prevention of colorectal cancer in people aged 40 or older: a systematic review and meta-analysis. Cancer Epidemiology. 2019;58:52–62.
- Liu Y, Jin PP, Sun XC, Hu TT. Thiazolidinediones and risk of colorectal cancer in patients with diabetes mellitus: a meta-analysis. Saudi Journal of Gastroenterol. 2018 Mar-Apr;24(2):75-81.
- Augustin Y, Krishna S, Kumar D, Pantziarka P. The wisdom of crowds and the repurposing of artesunate as an anticancer drug. Ecancermedicalscience. 2015;9:ed50.
- Katona BW, Weiss JM. Chemoprevention of colorectal cancer. Gastroenterology. 2020;158(2):368–388; Joo MK, Park JJ, Chun HJ. Additional benefits of routine drugs on gastrointestinal cancer: statins, metformin, and proton pump inhibitors. Digestive Diseases. 2018;36(1):1-14; Dobrzycka M, Spychalski P et al. Statins and colorectal cancer—a systematic review. Experimental and Clinical Endocrinology & Diabetes. 2018;10.1055/a-0668-5692; Qu B, Qu H. The influence of statins on risk and patient survival in colorectal cancer. Journal of Clinical Gastroenterology. 2019;53(9):699–701; Krstic MN, Mijac DD, Popovic DD, Pavlovic Markovic A, Milosavljević T. General aspects of primary cancer prevention. Digestive Diseases. 2019;37(5):406–415; Lee JW, You NY et al. Statin use and site-specific risk of colorectal cancer in individuals with hypercholesterolemia from the National Health Insurance Service-National Health Screening Cohort (NHIS-HEALS). Nutrition, Metabolism & Cardiovascular Diseases. 2019;29(7):701–709; Ibáñez-Sanz G, Guinó E et al. Statin use and the risk of colorectal cancer in a population-based electronic health records study. Scientific Reports. 2019;9(1):13560; Lai SW, Kuo YH, Fang CW, Liao KF. Statins therapy and colorectal cancer risk. Nutrition, Metabolism & Cardiovascular Diseases. 2019;29(12):1429–1430; Cheung KS, Chen L et al. Statins reduce the progression of non-advanced adenomas to colorectal cancer: a postcolonoscopy study in 187 897 patients. Gut. 2019;68(11):1979–1985; Bonovas S. Statins: do they have a potential role in cancer prevention and modifying cancer-related outcomes? Drugs. 2014 Oct;74(16):1841-8; Gronich N, Rennert G. Beyond aspirin--cancer prevention with statins, metformin and bisphosphonates. Nature Reviews. Clinical Oncology. 2013 Nov;10(11):625-42; Pisanti S, Picardi P, Ciaglia E, D'Alessandro A, Bifulco M. Novel prospects of statins as therapeutic agents in cancer. Pharmacological Research. 2014 Oct;88:84-98; Broughton T, Sington J, Beales IL. Statin use is associated with a reduced incidence of colorectal cancer: a colonoscopy-controlled case-control study. BMC Gastroenterology. 2012 Apr 24;12:36; Lytras T, Nikolopoulos G, Bonovas S. Statins and the risk of colorectal cancer: an updated systematic review and meta-analysis of 40 studies. World Journal of Gastroenterology. 2014 Feb 21;20(7):1858-70; Lochhead P, Chan AT. Statins and colorectal cancer. Clinical Gastroenterology and Hepatology. 2013 Feb;11(2):109-18; quiz e13-4\; Bowles EJA, Yu O et al. Cardiovascular medication use and risks of colon cancer recurrences and additional cancer events: a cohort study. BMC Cancer. 2019;19(1):270; Dolejs SC, Gayed B, Fajardo A. Prevention of colorectal neoplasia. Clinics in Colon and Rectal Surgery. 2016;29(4):353-362; Wakeman C, Keenan J et al. Chemoprevention of colorectal neoplasia. ANZ Journal of Surgery. 2017 Dec;87(12):E228-E232; Chae YK, Yousaf M et al. Statins as anti-cancer therapy; can we translate preclinical and epidemiologic data into clinical benefit? Discovery Medicine. 2015 Dec;20(112):413-27; Coogan PF, Smith J, Rosenberg L. Statin use and risk of colorectal cancer. Journal of the National Cancer Institute. 2007;99(1):32–40; Singh H, Mahmud SM, Turner D, Xue L, Demers AA, Bernstein CN. Long-term use of statins and risk of colorectal cancer: a population-based study. American Journal of Gastroenterology. 2009 Dec;104(12):3015-23.
- Katona BW, Weiss JM. Chemoprevention of colorectal cancer. Gastroenterology. 2020;158(2):368–388; Qu B, Qu H. The influence of statins on risk and patient survival in colorectal cancer. Journal of Clinical Gastroenterology. 2019;53(9):699–701; Liu Y, Tang W et al. Association between statin use and colorectal cancer risk: a meta-analysis of 42 studies. Cancer Causes Control. 2014 Feb;25(2):237-49; Pisanti S, Picardi P, Ciaglia E, D'Alessandro A, Bifulco M. Novel prospects of statins as therapeutic agents in cancer. Pharmacological Research. 2014 Oct;88:84-98; Broughton T, Sington J, Beales IL. Statin use is associated with a reduced incidence of colorectal cancer: a colonoscopy-controlled case-control study. BMC Gastroenterology. 2012 Apr 24;12:36; Samadder NJ, Mukherjee B et al. Risk of colorectal cancer in self-reported inflammatory bowel disease and modification of risk by statin and NSAID use. Cancer. 2011 Apr 15;117(8):1640-8.
- Qu B, Qu H. The influence of statins on risk and patient survival in colorectal cancer. Journal of Clinical Gastroenterology. 2019;53(9):699–701.
- Katona BW, Weiss JM. Chemoprevention of colorectal cancer. Gastroenterology. 2020;158(2):368–388.
- Chang R. Beyond the Magic Bullet: The Anti-Cancer Cocktail. New York: Square One Publishers. 2012.
- Yoshida K, Hashimoto T, Sakai Y, Hashiramoto A. Involvement of the circadian rhythm and inflammatory cytokines in the pathogenesis of rheumatoid arthritis. Journal of Immunology Research. 2014;2014:282495.
- Rich T, Innominato PF et al. Elevated serum cytokines correlated with altered behavior, serum cortisol rhythm, and dampened 24-hour rest-activity patterns in patients with metastatic colorectal cancer. Clinical Cancer Research. 2005 Mar 1;11(5):1757-64.
- Ishikawa H, Saeki T et al. Aged garlic extract prevents a decline of NK cell number and activity in patients with advanced cancer. Journal of Nutrition. 2006 Mar;136(3 Suppl):816S-820S.
- Rahman K, Billington D. Dietary supplementation with aged garlic extract inhibits ADP-induced platelet aggregation in humans. Journal of Nutrition. 2000 Nov;130(11):2662-5.
- Silagy CA, Neil HA. A meta-analysis of the effect of garlic on blood pressure. Journal of Hypertension. 1994 Apr;12(4):463-8.
- Ota A, Ulrih NP. An overview of herbal products and secondary metabolites used for management of type two diabetes. Frontiers in Pharmacology. 2017 Jul 6;8:436.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Ong SKL, Shanmugam MK et al. Focus on formononetin: anticancer potential and molecular targets. Cancers (Basel). 2019;11(5):611; Dong J, Liang W et al. Saponins regulate intestinal inflammation in colon cancer and IBD. Pharmacological Research. 2019;144:66–72.
- Chen M, May BH, Zhou IW, Xue CC, Zhang AL. FOLFOX 4 combined with herbal medicine for advanced colorectal cancer: a systematic review. Phytotherapy Research. 2014;28(7):976–991; Chen M, May BH et al. Oxaliplatin-based chemotherapy combined with traditional medicines for neutropenia in colorectal cancer: a meta-analysis of the contributions of specific plants. Critical Reviews in Oncology/Hematology. 2016;105:18–34; Dong J, Liang W et al. Saponins regulate intestinal inflammation in colon cancer and IBD. Pharmacological Research. 2019;144:66–72.
- Chen MH, May BH, Zhou IW, Zhang AL, Xue CC. Integrative medicine for relief of nausea and vomiting in the treatment of colorectal cancer using oxaliplatin-based chemotherapy: a systematic review and meta-analysis. Phytotherapy Research. 2016;30(5):741–753; Dong J, Liang W et al. Saponins regulate intestinal inflammation in colon cancer and IBD. Pharmacological Research. 2019;144:66–72.
- Wang QZ, Chen XP, Huang JP, Jiang XW. [Effects of couplet medicines (Astragalus membranaceus and jiaozhen) on intestinal barrier in postoperative colorectal cancer patients] [Article in Chinese]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2015;35(11):1307–1312.
- Yoshikawa K, Shimada M et al. The effects of the Kampo medicine (Japanese herbal medicine) "Daikenchuto" on the surgical inflammatory response following laparoscopic colorectal resection. Surgery Today. 2012 Jul;42(7):646-51.
- Chen D, Yang Y, Yang P. Quxie Capsule inhibits colon tumor growth partially through foxo1-mediated apoptosis and immune modulation. Integrative Cancer Therapies. 2019;18:1534735419846377; Yang YF, Xu Y, Wu Y. [Clinical randomized double-blinded controlled study on Quxie Capsule in reducing post-operational relapse and metastasis of colorectal cancer] [Article in Chinese]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2007;27(10):879–882.
- Lagoa R, Silva J, Rodrigues JR, Bishayee A. Advances in phytochemical delivery systems for improved anticancer activity. Biotechnology Advances. 2019 Apr 9. pii: S0734-9750(19)30063-1; Amalraj A, Pius A, Gopi S, Gopi S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—a review. Journal of Traditional and Complementary Medicine. 2016 Jun 15;7(2):205-233; Xu B, Yu L, Zhao LZ. Curcumin up regulates T helper 1 cells in patients with colon cancer. American Journal of Translational Research. 2017 Apr 15;9(4):1866-1875; Núñez-Sánchez MA, González-Sarrías A et al. Dietary phenolics against colorectal cancer—from promising preclinical results to poor translation into clinical trials: pitfalls and future needs. Molecular Nutrition & Food Research. 2015 Jul;59(7):1274-91; Hou TY, Davidson LA et al. Nutrient-gene interaction in colon cancer, from the membrane to cellular physiology. Annual Review of Nutrition. 2016 Jul 17;36:543-70; Panahi Y, Saadat A, Beiraghdar F, Sahebkar A. Adjuvant therapy with bioavailability-boosted curcuminoids suppresses systemic inflammation and improves quality of life in patients with solid tumors: a randomized double-blind placebo-controlled trial. Phytotherapy Research. 2014 Oct;28(10):1461-7; Fadus MC, Lau C, Bikhchandani J, Lynch HT. Curcumin: an age-old anti-inflammatory and anti-neoplastic agent. Journal of Traditional and Complementary Medicine. 2016 Sep 9;7(3):339-346; Das J, Ramani R, Suraju MO. Polyphenol compounds and PKC signaling. Biochimica et Biophysica Acta. 2016 Oct;1860(10):2107-21.
- Panahia Y, Saadat A et al. Antioxidant effects of bioavailability-enhanced curcuminoids in patients with solid tumors: a randomized double-blind placebo-controlled trial. Journal of Functional Foods. 2014 Jan;6:615-622.
- Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS Journal. 2013 Jan;15(1):195-218; Carroll RE, Benya RV et al. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prevention Research (Philadelphia, Pennsylvania). 2011 Mar;4(3):354-64.
- He ZY, Shi CB et al. Upregulation of p53 expression in patients with colorectal cancer by administration of curcumin. Cancer Investigation. 2011 Mar;29(3):208-13; Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS Journal . 2013 Jan;15(1):195-218; Carroll RE, Benya RV et al. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prevention Research (Philadelphia, Pennsylvania). 2011 Mar;4(3):354-64. Imran M, Ullah A et al. Cucurmin, anticancer, & antitumor perspectives: a comprehensive review. Critical Reviews in Food Science and Nutrition. 2018 May 24;58(8):1271-1293; Pabla B, Bissonnette M, Konda VJ. Colon cancer and the epidermal growth factor receptor: current treatment paradigms, the importance of diet, and the role of chemoprevention. World Journal of Clinical Oncology. 2015 Oct 10;6(5):133-41; Hou TY, Davidson LA et al. Nutrient-gene interaction in colon cancer, from the membrane to cellular physiology. Annual Review of Nutrition. 2016 Jul 17;36:543-70.
- Lagoa R, Silva J, Rodrigues JR, Bishayee A. Advances in phytochemical delivery systems for improved anticancer activity. Biotechnology Advances. 2019 Apr 9. pii: S0734-9750(19)30063-1.
- McFadden RM, Larmonier CB et al. The role of curcumin in modulating colonic microbiota during colitis and colon cancer prevention. Inflammatory Bowel Diseases. 2015 Nov;21(11):2483-94.
- Alschuler LN, Gazella KA. The Definitive Guide to Cancer, 3rd Edition: An Integrative Approach to Prevention, Treatment, and Healing. Berkeley, California: Celestial Arts. 2010; Alschuler LN, Gazella KA. The Definitive Guide to Thriving after Cancer: A Five-Step Integrative Plan to Reduce the Risk of Recurrence and Build Lifelong Health. Berkeley, California: Ten Speed Press. 2013.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Mueller T, Voigt W. Fermented wheat germ extract—nutritional supplement or anticancer drug? Nutrition Journal. 2011 Sep 5;10:89.
- Mueller T, Voigt W. Fermented wheat germ extract—nutritional supplement or anticancer drug? Nutrition Journal. 2011 Sep 5;10:89; Telekes A, Hegedus M, Chae CH, Vékey K. Avemar (wheat germ extract) in cancer prevention and treatment. Nutrition and Cancer. 2009;61(6):891-9.
- Núñez-Sánchez MA, González-Sarrías A et al. Dietary phenolics against colorectal cancer—from promising preclinical results to poor translation into clinical trials: pitfalls and future needs. Molecular Nutrition and Food Research. 2015 Jul;59(7):1274-91; Alsherbiny MA, Abd-Elsalam WH et al. Ameliorative and protective effects of ginger and its main constituents against natural, chemical and radiation-induced toxicities: a comprehensive review. Food and Chemical Toxicology. 2019 Jan;123:72-97; López-Romero D, Izquierdo-Vega JA. Evidence of some natural products with antigenotoxic effects. part 2: plants, vegetables, and natural resin. Nutrients. 2018 Dec 10;10(12). pii: E1954; Mahomoodally MF, Aumeeruddy MZ et al. Ginger and its active compounds in cancer therapy: from folk uses to nano-therapeutic applications. Seminars in Cancer Biology. 2019 Aug 11. pii: S1044-579X(19)30213-5.
- de Lima RMT, Dos Reis AC et al. Protective and therapeutic potential of ginger (Zingiber officinale) extract and [6]-gingerol in cancer: a comprehensive review. Phytotherapy Research. 2018 Oct;32(10):1885-1907; Núñez-Sánchez MA, González-Sarrías A et al. Dietary phenolics against colorectal cancer—from promising preclinical results to poor translation into clinical trials: pitfalls and future needs. Molecular Nutrition and Food Research. 2015 Jul;59(7):1274-91; Alsherbiny MA, Abd-Elsalam WH et al. Ameliorative and protective effects of ginger and its main constituents against natural, chemical and radiation-induced toxicities: a comprehensive review. Food and Chemical Toxicology. 2019 Jan;123:72-97; Kim Y, Kim DM, Kim JY. Ginger extract suppresses inflammatory response and maintains barrier function in human colonic epithelial caco-2 cells exposed to inflammatory mediators. Journal of Food Science. 2017 May;82(5):1264-1270; Mahomoodally MF, Aumeeruddy MZ et al. Ginger and its active compounds in cancer therapy: from folk uses to nano-therapeutic applications. Seminars in Cancer Biology. 2019 Aug 11. pii: S1044-579X(19)30213-5
- Zhang M, Viennois E et al. Edible ginger-derived nanoparticles: a novel therape utic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials. 2016 Sep;101:321-40.
- Kim Y, Kim DM, Kim JY. Ginger extract suppresses inflammatory response and maintains barrier function in human colonic epithelial caco-2 cells exposed to inflammatory mediators. Journal of Food Science. 2017 May;82(5):1264-1270.
- Mahomoodally MF, Aumeeruddy MZ et al. Ginger and its active compounds in cancer therapy: from folk uses to nano-therapeutic applications. Seminars in Cancer Biology. 2019 Aug 11. pii: S1044-579X(19)30213-5.
- Almatroudi A, Alsahli MA, Alrumaihi F, Allemailem KS, Rahmani AH. Ginger: a novel strategy to battle cancer through modulating cell signalling pathways: a review. Current Pharmaceutical Biotechnology. 2019;20(1):5-16.
- Wee LH, Morad NA et al. Mechanism of chemoprevention against colon cancer cells using combined gelam honey and ginger extract via mTOR and Wnt/β-catenin pathways. Asian Pacific Journal of Cancer Prevention. 2015;16(15):6549-56.
- Alschuler LN, Gazella KA. The Definitive Guide to Cancer, 3rd Edition: An Integrative Approach to Prevention, Treatment, and Healing. Berkeley, California: Celestial Arts. 2010; Alschuler LN, Gazella KA. The Definitive Guide to Thriving after Cancer: A Five-Step Integrative Plan to Reduce the Risk of Recurrence and Build Lifelong Health. Berkeley, California: Ten Speed Press. 2013.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- McKinney N. Naturopathic Oncology, 3rd Edition. Victoria, BC, Canada: Liaison Press. 2016.
- de Urbina JJO, San-Miguel B et al. Effects of oral glutamine on inflammatory and autophagy responses in cancer patients treated with abdominal radiotherapy: a pilot randomized trial. International Journal of Medical Sciences. 2017 Sep 4;14(11):1065-1071.
- Rotovnik Kozjek N, Kompan L, Žagar T, Mrevlje Ž. Influence of enteral glutamine on inflammatory and hormonal response in patients with rectal cancer during preoperative radiochemotherapy. European Journal of Clinical Nutrition. 2017;71(5):671-673.
- Jolfaie NR, Mirzaie S, Ghiasvand R, Askari G, Miraghajani M. The effect of glutamine intake on complications of colorectal and colon cancer treatment: a systematic review. Journal of Research in Medical Sciences. 2015 Sep;20(9):910-8.
- van Stijn MFM, Soeters MR et al. Effects of a carbohydrate-, glutamine-, and antioxidant-enriched oral nutrition supplement on major surgery-induced insulin resistance: a randomized pilot study. JPEN. Journal of Parenteral and Enteral Nutrition. 2018 May;42(4):719-729.
- Cui Y, Hu L et al. Intravenous alanyl-L-glutamine balances glucose-insulin homeostasis and facilitates recovery in patients undergoing colonic resection: a randomised controlled trial. European Journal of Anaesthesiology. 2014 Apr;31(4):212-8.
- Szpetnar M, Matras P et al. Is additional enrichment of diet in branched-chain amino acids or glutamine beneficial for patients receiving total parenteral nutrition after gastrointestinal cancer surgery? Advances in Clinical and Experimental Medicine. 2014 May-Jun;23(3):423-31.
- Jin JW, Inoue O et al. Grape seed extracts inhibit platelet aggregation by inhibiting protein tyrosine phosphatase. Clinical and Applied Thrombosis/Hemostasis. Apr 2014;20(3):278-284; Bijak M, Bobrowski M et al. Anticoagulant effect of polyphenols-rich extracts from black chokeberry and grape seeds. Fitoterapia. 2011 Sep;82(6):811-7; de Lange DW, Scholman WL, Kraaijenhagen RJ, Akkerman JW, van de Wiel A. Alcohol and polyphenolic grape extract inhibit platelet adhesion in flowing blood. European Journal of Clinical Investigation. 2004 Dec;34(12):818-24; de Lange DW, Verhoef S et al. Polyphenolic grape extract inhibits platelet activation through PECAM-1: an explanation for the French paradox. Alcoholism, Clinical and Experimental Research. 2007 Aug;31(8):1308-14.
- Derry MM, Raina K, Agarwal C, Agarwal R. Identifying molecular targets of lifestyle modifications in colon cancer prevention. Frontiers in Oncology. 2013 May 14;3:119; Katiyar SK, Athar M. Grape seeds: ripe for cancer chemoprevention. Cancer Prevention Research (Philadelphin, PA). 2013 Jul;6(7):617-21.
- Derry MM, Raina K et al. Grape seed extract efficacy against azoxymethane-induced colon tumorigenesis in A/J mice: interlinking miRNA with cytokine signaling and inflammation. Cancer Prevention Research (Philadelphia). 2013 Jul;6(7):625-33; Derry MM, Raina K, Agarwal C, Agarwal R. Identifying molecular targets of lifestyle modifications in colon cancer prevention. Frontiers in Oncology. 2013 May 14;3:119.
- Katiyar SK, Athar M. Grape seeds: ripe for cancer chemoprevention. Cancer Prevention Research (Philadelphin, PA). 2013 Jul;6(7):617-21.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Ni J, Guo X, Wang H, Zhou T, Wang X. Differences in the effects of EGCG on chromosomal stability and cell growth between normal and colon cancer cells. Molecules. 2018 Mar 29;23(4). pii: E788; Carini F, Tomasello G et al. Colorectal cancer and inflammatory bowel diseases: effects of diet and antioxidants. Journal of Biological Regulators and Homeostatic Agents. 2017 Jul-Sep;31(3):791-795; Kuppusamy P, Yusoff MM et al. Nutraceuticals as potential therapeutic agents for colon cancer: a review. Acta Pharm Sin B. 2014 Jun;4(3):173-81; Fajardo AM, Piazza GA. Chemoprevention in gastrointestinal physiology and disease. Anti-inflammatory approaches for colorectal cancer chemoprevention. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2015 Jul 15;309(2):G59-70; Massounga Bora AF, Ma S, Li X, Liu L Application of microencapsulation for the safe delivery of green tea polyphenols in food systems: review and recent advances. Food Research International. 2018 Mar;105:241-249.
- Liu C, Li P et al. Advances in the antagonism of epigallocatechin-3-gallate in the treatment of digestive tract tumors. Molecules. 2019 May 3;24(9). pii: E1726; Fajardo AM, Piazza GA. Chemoprevention in gastrointestinal physiology and disease. Anti-inflammatory approaches for colorectal cancer chemoprevention. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2015 Jul 15;309(2):G59-70; Shirakami Y, Ohnishi M, Sakai H, Tanaka T, Shimizu M. Prevention of colorectal cancer by targeting obesity-related disorders and inflammation. International Journal of Molecular Sciences. 2017 Apr 26;18(5). pii: E908.
- Massounga Bora AF, Ma S, Li X, Liu L Application of microencapsulation for the safe delivery of green tea polyphenols in food systems: review and recent advances. Food Research International. 2018 Mar;105:241-249.
- Ni J, Guo X, Wang H, Zhou T, Wang X. Differences in the effects of EGCG on chromosomal stability and cell growth between normal and colon cancer cells. Molecules. 2018 Mar 29;23(4). pii: E788.
- Fajardo AM, Piazza GA. Chemoprevention in gastrointestinal physiology and disease. Anti-inflammatory approaches for colorectal cancer chemoprevention. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2015 Jul 15;309(2):G59-70.
- Zheng XX, Xu YL et al. Effects of green tea catechins with or without caffeine on glycemic control in adults: a meta-analysis of randomized controlled trials. American Journal of Clinical Nutrition. 2013 Apr;97(4):750-62; Kim Y, Keogh JB, Clifton PM. Polyphenols and glycemic control. Nutrients. 2016 Jan 5;8(1). pii: E17; Liu K, Zhou R et al. Effect of green tea on glucose control and insulin sensitivity: a meta-analysis of 17 randomized controlled trials. American Journal of Clinical Nutrition. 2013 Aug;98(2):340-8.
- Yu J, Song P, Perry R, Penfold C, Cooper AR. The effectiveness of green tea or green tea extract on insulin resistance and glycemic control in type 2 diabetes mellitus: a meta-analysis. Diabetes & Metabolism Journal. 2017 Aug;41(4):251-262.
- Alschuler LN, Gazella KA. The Definitive Guide to Cancer, 3rd Edition: An Integrative Approach to Prevention, Treatment, and Healing. Berkeley, California: Celestial Arts. 2010; Alschuler LN, Gazella KA. The Definitive Guide to Thriving after Cancer: A Five-Step Integrative Plan to Reduce the Risk of Recurrence and Build Lifelong Health. Berkeley, California: Ten Speed Press. 2013.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- McKinney N. Naturopathic Oncology, 3rd Edition. Victoria, BC, Canada: Liaison Press. 2016.
- Yehia R, Saleh S, El Abhar H, Saad AS, Schaalan M. L-Carnosine protects against oxaliplatin-induced peripheral neuropathy in colorectal cancer patients: a perspective on targeting Nrf-2 and NF-κB pathways. Toxicology and Applied Pharmacology. 2019;365:41-50.
- Zhong Z, Wheeler MD et al. L-glycine: a novel antiinflammatory, immunomodulatory, and cytoprotective agent. Current Opinions in Clinical Nutrition and Metabolic Care. 2003 Mar;6(2):229-40.
- Pappalardo G, Almeida A, Ravasco P. Eicosapentaenoic acid in cancer improves body composition and modulates metabolism. Nutrition. 2015 Apr;31(4):549-55; de Aguiar Pastore Silva J, Emilia de Souza Fabre M, Waitzberg DL. Omega-3 supplements for patients in chemotherapy and/or radiotherapy: a systematic review. Clinical Nutrition. 2015 Jun;34(3):359-66.
- Sorensen LS, Thorlacius-Ussing O et al. Effects of perioperative supplementation with omega-3 fatty acids on leukotriene B₄ and leukotriene B₅ production by stimulated neutrophils in patients with colorectal cancer: a randomized, placebo-controlled intervention trial. Nutrients. 2014 Sep 29;6(10):4043-57; Silva Jde A, Trindade EB et al. Fish oil supplement alters markers of inflammatory and nutritional status in colorectal cancer patients. Nutrition and Cancer. 2012;64(2):267-73; Xie H, Chang YN. Omega-3 polyunsaturated fatty acids in the prevention of postoperative complications in colorectal cancer: a meta-analysis. OncoTargets and Therapy. 2016 Dec 9;9:7435-7443; Golkhalkhali B, Rajandram R et al. Strain-specific probiotic (microbial cell preparation) and omega-3 fatty acid in modulating quality of life and inflammatory markers in colorectal cancer patients: a randomized controlled trial. Asia-Pacific Journal of Clinical Oncology. 2018 Jun;14(3):179-191.
- Mocellin MC, Camargo CQ, Nunes EA, et al. A systematic review and meta-analysis of the n-3 polyunsaturated fatty acids effects on inflammatory markers in colorectal cancer. Clinical Nutrition. 2016;35:359–369.
- Liang B, Wang S, Impact of postoperative omega-3 fatty acid-supplemented parenteral nutrition on clinical outcomes and immunomodulations in colorectal cancer patients. World Journal of Gastroenterology. 2008 Apr 21;14(15):2434-9.
- Aisu N, Tanimura S et al. Impact of perioperative probiotic treatment for surgical site infections in patients with colorectal cancer. Experimental and Therapeutic Medicine. 2015;10(3):966–972; Roller M, Clune Y, Collins K, Rechkemmer G, Watzl B. Consumption of prebiotic inulin enriched with oligofructose in combination with the probiotics Lactobacillus rhamnosus and Bifidobacterium lactis has minor effects on selected immune parameters in polypectomised and colon cancer patients. British Journal of Nutrition. 2007;97(4):676–684; Anderson SW, Bazzell AF, Dains JE. An integrative review on the effect of prebiotics, probiotics, and synbiotics on infection after colorectal cancer surgery. AORN Journal. 2018;107(2):237–248.
- Aisu N, Tanimura S et al. Impact of perioperative probiotic treatment for surgical site infections in patients with colorectal cancer. Experimental and Therapeutic Medicine. 2015;10(3):966–972.
- Nio Y, Tsubono M et al. Immunomodulation by orally administered protein-bound polysaccharide Krestin (PSK)™ in patients with gastrointestinal cancer. Biotherapy. 1992;4:117–128.
- Nio Y, Tsubono M et al. Immunomodulation by orally administered protein-bound polysaccharide PSK in patients with gastrointestinal cancer. Biotherapy. 1992;4(2):117-28; Evidence-Based Monographs: Professional Resource: Coriolus Versicolor. Ottawa Integrative Cancer Centre. Viewed June 6, 2019.
- Mayland CR, Bennett MI, Allan K. Vitamin C deficiency in cancer patients. Palliative Medicine.2005;19:17–20. with improvements seen after intravenous vitamin C treatmentMikirova N, Casciari J, Riordan N, Hunninghake R. Clinical experience with intravenous administration of ascorbic acid: achievable levels in blood for different states of inflammation and disease in cancer patients. Journal of Translational Medicine. 2013;11:191.
- Hanson MG, Ozenci V et al. A short-term dietary supplementation with high doses of vitamin E increases NK cell cytolytic activity in advanced colorectal cancer patients. Cancer Immunology, Immunotherapy. 2007;56(7):973-984; Malmberg KJ, Lenkei R et al. A short-term dietary supplementation of high doses of vitamin E increases T helper 1 cytokine production in patients with advanced colorectal cancer. Clinical Cancer Research. 2002 Jun;8(6):1772-8.
- Lasry A, Zinger A, Ben-Neriah Y. Inflammatory networks underlying colorectal cancer. Nature Immunology. 2016 Mar;17(3):230-40; Grancher A, Michel P, Di Fiore F, Sefrioui D. [Aspirin and colorectal cancer]. Article in French. Bulletin du Cancer. 2018 Feb;105(2):171-180; Pan P, Huang YW et al. Could aspirin and diets high in fiber act synergistically to reduce the risk of colon cancer in humans? International Journal of Molecular Sciences. 2018 Jan 6;19(1). pii: E166; Chen J, Stark LA. Aspirin prevention of colorectal cancer: focus on NF-κB signalling and the nucleolus. Biomedicines. 2017 Jul 18;5(3). pii: E43.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Bader JE, Enos RT, Velázquez KT, et al. Macrophage depletion using clodronate liposomes decreases tumorigenesis and alters gut microbiota in the AOM/DSS mouse model of colon cancer. American Journal of Physiology—Gastrointestinal and Liver Physiology. 2018;314(1):G22–G31.
- Bader JE, Enos RT, Velázquez KT, et al. Macrophage depletion using clodronate liposomes decreases tumorigenesis and alters gut microbiota in the AOM/DSS mouse model of colon cancer. American Journal of Physiology—Gastrointestinal and Liver Physiology. 2018;314(1):G22–G31.
- Losurdo G, Principi M et al. Histamine and histaminergic receptors in colorectal cancer: from basic science to evidence-based medicine. Anti-Cancer Agents in Medicinal Chemistry. 2018;18(1):15–20; Pantziarka P, Bouche G, Meheus L, Sukhatme V, Sukhatme VP. Repurposing drugs in oncology (ReDO)—cimetidine as an anti-cancer agent. Ecancermedicalscience. 2014;8:485; Eaton D, Hawkins RE. Cimetidine in colorectal cancer--are the effects immunological or adhesion-mediated? British Journal of Cancer. 2002;86(2):159–160.
- Lin CY, Bai DJ, Yuan HY, et al. Perioperative cimetidine administration promotes peripheral blood lymphocytes and tumor infiltrating lymphocytes in patients with gastrointestinal cancer: results of a randomized controlled clinical trial. World Journal of Gastroenterology. 2004;10(1):136–142; Li B, Cao F, Zhu Q, et al. Perioperative cimetidine administration improves systematic immune response and tumor infiltrating lymphocytes in patients with colorectal cancer. Hepatogastroenterology 2013;60:244–247; Kumar A. Cimetidine: an immunomodulator. DICP. 1990 Mar;24(3):289-95.
- Eaton D, Hawkins RE. Cimetidine in colorectal cancer--are the effects immunological or adhesion-mediated? British Journal of Cancer. 2002;86(2):159–160.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Lemole G, Mehta P, McKee D. After Cancer Care: The Definitive Self-Care Guide to Getting and Staying Well for Patients with Cancer. New York, New York: Rodale, Inc. 2015.
- Brewer GJ. Anticopper therapy against cancer and diseases of inflammation and fibrosis. Drug Discovery Today. 2005;10(16):1103–1109.
- Khan G, Merajver S. Copper chelation in cancer therapy using tetrathiomolybdate: an evolving paradigm. Expert Opinion on Investigational Drugs. 2009;18(4):541–548.
- Joo MK, Park JJ, Chun HJ. Additional benefits of routine drugs on gastrointestinal cancer: statins, metformin, and proton pump inhibitors. Digestive Diseases. 2018;36(1):1-14.
- Umezawa S, Higurashi T et al. Chemoprevention of colorectal cancer: past, present, and future. Cancer Science. 2019 Oct;110(10):3018-3026.
- Cui G, Zhang T et al. High blood glucose levels correlate with tumor malignancy in colorectal cancer patients. Medical Science Monitor. 2015 Dec 8;21:3825-33; Perrigue MM, Drewnowski A et al. Randomized trial testing the effects of eating frequency on two hormonal biomarkers of metabolism and energy balance. Nutrition and Cancer. 2017 Jan;69(1):56-63.
- Wolpin BM, Meyerhardt JA et al. Insulin, the insulin-like growth factor axis, and mortality in patients with nonmetastatic colorectal cancer. Journal of Clinical Oncology. 2009 Jan 10;27(2):176-85; Sax AT, Jenkins DG et al. The insulin-like growth factor axis: a biological mechanism linking physical activity to colorectal cancer survival. Cancer Epidemiology. 2014 Aug;38(4):455-9; Yuan C, Bao Y et al. Influence of dietary insulin scores on survival in colorectal cancer patients. British Journal of Cancer. 2017 Sep 26;117(7):1079-1087.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- McKinney N. Naturopathic Oncology, 3rd Edition. Victoria, BC, Canada: Liaison Press. 2016.
- Lasry A, Zinger A, Ben-Neriah Y. Inflammatory networks underlying colorectal cancer. Nature Immunology. 2016;17(3):230–240.
- Tomić T, Domínguez-López S, Barrios-Rodríguez R. Non-aspirin non-steroidal anti-inflammatory drugs in prevention of colorectal cancer in people aged 40 or older: a systematic review and meta-analysis. Cancer Epidemiology. 2019;58:52–62.
- Buijsen J, van den Bogaard J et al. A phase I-II study on the combination of rapamycin and short course radiotherapy in rectal cancer. Radiotherapy & Oncology. 2015;116(2):214–220.
- Pisanti S, Picardi P, Ciaglia E, D'Alessandro A, Bifulco M. Novel prospects of statins as therapeutic agents in cancer. Pharmacological Research. 2014 Oct;88:84-98.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Pei X, Zhou Z, Xu G. [Effect of electroacupuncture for immune function of patients treated with laparoscopic radical rectectomy for rectal cancer] [Article in Chinese]. Zhongguo Zhen Jiu. 2016;36(6):613-616.
- Csiszar A, Labinskyy N, Jimenez R, et al. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: role of circulating factors and SIRT1. Mechanisms of Ageing and Development. 2009;130(8):518–527.
- Nencioni A, Caffa I, Cortellino S, Longo VD. Fasting and cancer: molecular mechanisms and clinical application. Nature Reviews. Cancer. 2018;18(11):707–719; Mansell PI, Macdonald IA. The effect of starvation on insulin-induced glucose disposal and thermogenesis in humans. Metabolism. 1990;39(5):502–510; Romijn JA, Godfried MH, Hommes MJ, Endert E, Sauerwein HP. Decreased glucose oxidation during short-term starvation. Metabolism. 1990;39(5):525–530; Ngo TH, Barnard RJ, Tymchuk CN, Cohen P, Aronson WJ. Effect of diet and exercise on serum insulin, IGF-I, and IGFBP-1 levels and growth of LNCaP cells in vitro (United States). Cancer Causes & Control. 2002;13(10):929–935.
- Ngo TH, Barnard RJ, Tymchuk CN, Cohen P, Aronson WJ. Effect of diet and exercise on serum insulin, IGF-I, and IGFBP-1 levels and growth of LNCaP cells in vitro (United States). Cancer Causes & Control. 2002;13(10):929–935; Sun P, Wang H, He Z, et al. Fasting inhibits colorectal cancer growth by reducing M2 polarization of tumor-associated macrophages. Oncotarget. 2017;8(43):74649–74660.
- Buono R, Longo VD. Starvation, stress resistance, and cancer. Trends in Endocrinology and Metabolism. 2018;29(4):271–280; Sun P, Wang H et al. Fasting inhibits colorectal cancer growth by reducing M2 polarization of tumor-associated macrophages. Oncotarget. 2017;8(43):74649–74660.
- Lu SSM, Mohammed Z et al. Antibiotics use and subsequent risk of colorectal cancer: a Swedish nationwide population-based study. JNCI: Journal of the National Cancer Institute. 2021 Sep 1:djab125.
- Zhang J, Haines C et al. Oral antibiotic use and risk of colorectal cancer in the United Kingdom, 1989-2012: a matched case-control study. Gut. 2019;68(11):1971–1978.
- Harrison P. Antibiotics use and increased risk of colon cancer? Medscape. August 23, 2019. Viewed February 25, 2020.
- Song M, Chan AT. Environmental factors, gut microbiota, and colorectal cancer prevention. Clinical Gastroenterology and Hepatology. 2019;17(2):275–289.
- Block KI, Block PB, Gyllenhaal C. Integrative treatment for colorectal cancer: a comprehensive approach. Journal of Alternative and Complementary Medicine. 2018;24(9-10):890–901.
- Block KI, Block PB, Gyllenhaal C. Integrative treatment for colorectal cancer: a comprehensive approach. Journal of Alternative and Complementary Medicine. 2018;24(9-10):890–901.
- Purcell RV, Pearson J et al. Colonization with enterotoxigenic Bacteroides fragilis is associated with early-stage colorectal neoplasia. PLoS One. 2017;12(2):e0171602.
- Block KI, Block PB, Gyllenhaal C. Integrative treatment for colorectal cancer: a comprehensive approach. Journal of Alternative and Complementary Medicine. 2018;24(9-10):890–901; Bultman SJ. Emerging roles of the microbiome in cancer. Carcinogenesis. 2014;35(2):249–255.
- Chattopadhyay I, Dhar R et al. Exploring the role of gut microbiome in colon cancer. Applied Biochemistry and Biotechnology. 2021 Jan 25; Dejea C, Wick E, Sears CL. Bacterial oncogenesis in the colon. Future Microbiology. 2013;8(4):445–460.
- Purcell RV, Pearson J et al. Colonization with enterotoxigenic Bacteroides fragilis is associated with early-stage colorectal neoplasia. PLoS One. 2017;12(2):e0171602.
- Melnyk M, Casey RG, Black P, Koupparis AJ. Enhanced recovery after surgery (ERAS) protocols: time to change practice? Canadian Urological Association Journal. 2011;5(5):342-348.
- Tebala GD, Gordon-Dixon A, Imtiaz M, Shrestha A, Toeima M. Enhanced recovery after rectal surgery: what we have learned so far. Mini-invasive Surgery. 2018;2:32.
- Tebala GD, Gordon-Dixon A, Imtiaz M, Shrestha A, Toeima M. Enhanced recovery after rectal surgery: what we have learned so far. Mini-invasive Surgery. 2018;2:32.
- Tebala GD, Gordon-Dixon A, Imtiaz M, Shrestha A, Toeima M. Enhanced recovery after rectal surgery: what we have learned so far. Mini-invasive Surgery. 2018;2:32; Calder PC. Immunonutrition. BMJ. 2003;327(7407):117-118.
- López Hellín J, Baena-Fustegueras JA, Sabín-Urkía P, Schwartz-Riera S, García-Arumí E. Nutritional modulation of protein metabolism after gastrointestinal surgery. European Journal of Clinical Nutrition. 2008;62(2):254-262.
- Gustafsson UO, Scott MJ et al. Guidelines for perioperative care in elective colorectal surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations: 2018. World Journal of Surgery. 2019;43(3):659-695.
- Ogbonna CI, Lembke A. Tapering patients off of benzodiazepines. American Family Physician. 2017 Nov 1;96(9):606-610.
- Carmichael JC, Keller DS et al. Clinical practice guidelines for enhanced recovery after colon and rectal surgery from the American Society of Colon and Rectal Surgeons and Society of American Gastrointestinal and Endoscopic Surgeons. Diseases of the Colon & Rectum. 2017;60(8):761-784.
- Minnella EM, Carli F. Prehabilitation and functional recovery for colorectal cancer patients. European Journal of Surgical Oncology. 2018;44(7):919-926.
- Minnella EM, Carli F. Prehabilitation and functional recovery for colorectal cancer patients. European Journal of Surgical Oncology. 2018;44(7):919-926.
- Kim SY, Kim NK et al. Effects of postoperative pain management on immune function after laparoscopic resection of colorectal cancer: a randomized study. Medicine (Baltimore). 2016 Jun 17;95(24):e4641.
- Haldar R, Ben-Eliyahu S. Reducing the risk of post-surgical cancer recurrence: a perioperative anti-inflammatory anti-stress approach. Future Oncology. 2018;14(11):1017-1021; Khanna AK, Perez ER, Laudanski K, Moraska A, Cummings KC. Perioperative care and cancer recurrence: Is there a connection? World Journal of Anesthesiology. 2014;3(1):31-45; Hiller JG, Perry NJ, Poulogiannis G, Riedel B, Sloan EK. Perioperative events influence cancer recurrence risk after surgery. Nature Reviews Clinical Oncology. 2018 Apr;15(4):205-218; Dang Y, Shi X, Xu W, Zuo M. The effect of anesthesia on the immune system in colorectal cancer patients. Canadian Journal of Gastroenterology & Hepatology. 2018 Apr 1;2018:7940603.
- Hiller JG, Perry NJ, Poulogiannis G, Riedel B, Sloan EK. Perioperative events influence cancer recurrence risk after surgery. Nature Reviews. Clinical Oncology. 2018;15(4):205-218.
- Sikorszki L, Bezsilla J, Vajda K, Temesi R. Lokálisan előrehaladott kemoirradiált rectumtumorok nyitott és laparoszkópos műtéteinek az összehasonlítása [The comparison of open and laparoscopic surgeries of the locally advanced chemo-irradiated rectum tumours]. Magyar Sebészet. 2020;73(1):29-36.
- Khanna AK, Perez ER, Laudanski K, Moraska A, Cummings KC. Perioperative care and cancer recurrence: Is there a connection? World Journal of Anesthesiology. 2014;3(1):31-45; Dang Y, Shi X, Xu W, Zuo M. The effect of anesthesia on the immune system in colorectal cancer patients. Canadian Journal of Gastroenterology & Hepatology. 2018 Apr1;2018:7940603.
- Dang Y, Shi X, Xu W, Zuo M. The effect of anesthesia on the immune system in colorectal cancer patients. Canadian Journal of Gastroenterology & Hepatology. 2018 Apr 1;2018:7940603; Gelman D, Gelmanas A et al. Role of multimodal analgesia in the evolving enhanced recovery after surgery pathways. Medicina. 2018 May; 54(2): 20.
- Khanna AK, Perez ER, Laudanski K, Moraska A, Cummings KC. Perioperative care and cancer recurrence: Is there a connection? World Journal of Anesthesiology. 2014;3(1):31-45.
- Khanna AK, Perez ER, Laudanski K, Moraska A, Cummings KC. Perioperative care and cancer recurrence: Is there a connection? World Journal of Anesthesiology. 2014;3(1):31-45; Hiller JG, Perry NJ, Poulogiannis G, Riedel B, Sloan EK. Perioperative events influence cancer recurrence risk after surgery. Nature Reviews Clinical Oncology. 2018 Apr;15(4):205-218; Dang Y, Shi X, Xu W, Zuo M. The Effect of Anesthesia on the Immune System in Colorectal Cancer Patients. Canadian Journal of Gastroenterology & Hepatology. 2018 Apr 1;2018:7940603.
- Khanna AK, Perez ER, Laudanski K, Moraska A, Cummings KC. Perioperative care and cancer recurrence: Is there a connection? World Journal of Anesthesiology. 2014;3(1):31-45; Hiller JG, Perry NJ, Poulogiannis G, Riedel B, Sloan EK. Perioperative events influence cancer recurrence risk after surgery. Nature Reviews Clinical Oncology. 2018 Apr;15(4):205-218; Dang Y, Shi X, Xu W, Zuo M. The effect of anesthesia on the immune system in colorectal cancer patients. Canadian Journal of Gastroenterology & Hepatology. 2018 Apr 1;2018:7940603.
- Richards CH, Platt JJ et al. The impact of perioperative risk, tumor pathology and surgical complications on disease recurrence following potentially curative resection of colorectal cancer. Annals of Surgery. 2011 Jul;254(1):83-9.
- Dang Y, Shi X, Xu W, Zuo M. The effect of anesthesia on the immune system in colorectal cancer patients. Canadian Journal of Gastroenterology & Hepatology. 2018 Apr 1;2018:7940603; Gelman D, Gelmanas A et al. Role of multimodal analgesia in the evolving enhanced recovery after surgery pathways. Medicina. 2018 May; 54(2): 20.
- Fligor SC, Wang S, Allar BG, et al. Gastrointestinal malignancies and the COVID-19 pandemic: evidence-based triage to surgery. Journal of Gastrointestinal Surgery. 2020;1-17.
- Haldar R, Ben-Eliyahu S. Reducing the risk of post-surgical cancer recurrence: a perioperative anti-inflammatory anti-stress approach. Future Oncology. 2018;14(11):1017-1021; Mills GA, Horn JR. Beta-blockers and glucose control. Drug Intelligence and Clinical Pharmacy. 1985 Apr;19(4):246-51.
- Hiller JG, Perry NJ, Poulogiannis G, Riedel B, Sloan EK. Perioperative events influence cancer recurrence risk after surgery. Nature Reviews Clinical Oncology. 2018 Apr;15(4):205-218.
- Aoyama T, Oba K et al. Impact of postoperative complications on the colorectal cancer survival and recurrence: analyses of pooled individual patients' data from three large phase III randomized trials. Cancer Medicine. 2017;6(7):1573‐1580; Kirchhoff P, Clavien PA, Hahnloser D. Complications in colorectal surgery: risk factors and preventive strategies. Patient Safety in Surgery. 2010;4(1):5.
- Aoyama T, Oba K et al. Impact of postoperative complications on the colorectal cancer survival and recurrence: analyses of pooled individual patients' data from three large phase III randomized trials. Cancer Medicine. 2017;6(7):1573‐1580; Kirchhoff P, Clavien PA, Hahnloser D. Complications in colorectal surgery: risk factors and preventive strategies. Patient Safety in Surgery. 2010;4(1):5; Aquina CT, Mohile SG et al. The impact of age on complications, survival, and cause of death following colon cancer surgery. British Journal of Cancer. 2017;116(3):389-397; Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Care. New York: Bantam Dell. 2009.
- Anderson SW, Bazzell AF, Dains JE. An integrative review on the effect of prebiotics, probiotics, and synbiotics on infection after colorectal cancer surgery. AORN Journal. 2018;107(2):237–248.
- Kim IY, Kim BR, Kim YW. Factors affecting use and delay (≥8 weeks) of adjuvant chemotherapy after colorectal cancer surgery and the impact of chemotherapy-use and delay on oncologic outcomes. PLoS One. 2015;10(9):e0138720.
- Cata JP, Guerra CE, Chang GJ, Gottumukkala V, Joshi GP. Non-steroidal anti-inflammatory drugs in the oncological surgical population: beneficial or harmful? A systematic review of the literature. British Journal of Anaesthesia. 2017;119(4):750-764.
- Aoyama T, Oba K et al. Impact of postoperative complications on the colorectal cancer survival and recurrence: analyses of pooled individual patients' data from three large phase III randomized trials. Cancer Medicine. 2017;6(7):1573‐1580.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Care. New York: Bantam Dell. 2009; Aquina CT, Mohile SG et al. The impact of age on complications, survival, and cause of death following colon cancer surgery. British Journal of Cancer. 2017;116(3):389-397.
- Kirchhoff P, Clavien PA, Hahnloser D. Complications in colorectal surgery: risk factors and preventive strategies. Patient Safety in Surgery. 2010;4(1):5.
- Minnella EM, Carli F. Prehabilitation and functional recovery for colorectal cancer patients. European Journal of Surgical Oncology. 2018;44(7):919‐926.
- Kirchhoff P, Clavien PA, Hahnloser D. Complications in colorectal surgery: risk factors and preventive strategies. Patient Safety in Surgery. 2010;4(1):5.
- Minnella EM, Carli F. Prehabilitation and functional recovery for colorectal cancer patients. European Journal of Surgical Oncology. 2018;44(7):919‐926.
- Holubar SD, Brickman RK, Greaves SW, Ivatury SJ. Neoadjuvant radiotherapy: a risk factor for short-term wound complications after radical resection for rectal cancer? Journal of the American College of Surgeons. 2016;223(2):291‐298.
- Schiffmann L, Wedermann N et al. Intensified neoadjuvant radiochemotherapy for rectal cancer enhances surgical complications. BMC Surgery. 2013;13:43.
- Rutegård J, Rutegård M. Non-steroidal anti-inflammatory drugs in colorectal surgery: a risk factor for anastomotic complications? World Journal of Gastrointestinal Surgery. 2012;4(12):278-280.
- Anderson SW, Bazzell AF, Dains JE. An integrative review on the effect of prebiotics, probiotics, and synbiotics on infection after colorectal cancer surgery. AORN Journal. 2018;107(2):237–248.
- Xiao J, Caan BJ et al. Association of low muscle mass and low muscle radiodensity with morbidity and mortality for colon cancer surgery. JAMA Surgery. 2020;e202497.
- Cata JP, Guerra CE, Chang GJ, Gottumukkala V, Joshi GP. Non-steroidal anti-inflammatory drugs in the oncological surgical population: beneficial or harmful? A systematic review of the literature. British Journal of Anaesthesia. 2017;119(4):750-764; Rutegård J, Rutegård M. Non-steroidal anti-inflammatory drugs in colorectal surgery: a risk factor for anastomotic complications? World Journal of Gastrointestinal Surgery. 2012;4(12):278-280.
- Phillips B. Reducing gastrointestinal anastomotic leak rates: review of challenges and solutions. Open Access Surgery. 2016;9:5-14; Rutegård J, Rutegård M. Non-steroidal anti-inflammatory drugs in colorectal surgery: a risk factor for anastomotic complications? World Journal of Gastrointestinal Surgery. 2012;4(12):278-280; Kendrick JB, Kaye AD et al. Goal-directed fluid therapy in the perioperative setting. Journal of Anaesthesiology and Clinical Pharmacology. 2019 Apr;35(Suppl 1):S29-S34.
- Cho JS, Lee MH et al. The effects of perioperative anesthesia and analgesia on immune function in patients undergoing breast cancer resection: a prospective randomized study. International Journal of Medical Sciences. 2017 Aug 18;14(10):970-976.
- Kim IY, Kim BR, Kim YW. Factors affecting use and delay (≥8 weeks) of adjuvant chemotherapy after colorectal cancer surgery and the impact of chemotherapy-use and delay on oncologic outcomes. PLoS One. 2015;10(9):e0138720.
- Schiffmann L, Wedermann N et al. Intensified neoadjuvant radiochemotherapy for rectal cancer enhances surgical complications. BMC Surgery. 2013;13:43.
- Holubar SD, Brickman RK, Greaves SW, Ivatury SJ. Neoadjuvant radiotherapy: a risk factor for short-term wound complications after radical resection for rectal cancer? Journal of the American College of Surgeons. 2016;223(2):291‐298.
- O'Gorman C, Denieffe S, Gooney M. Literature review: preoperative radiotherapy and rectal cancer—impact on acute symptom presentation and quality of life. Journal of Clinical Nursing. 2014;23(3-4):333‐351.
- Schiffmann L, Wedermann N et al. Intensified neoadjuvant radiochemotherapy for rectal cancer enhances surgical complications. BMC Surgery. 2013;13:43.
- Sikorszki L, Bezsilla J, Vajda K, Temesi R. Lokálisan előrehaladott kemoirradiált rectumtumorok nyitott és laparoszkópos műtéteinek az összehasonlítása [The comparison of open and laparoscopic surgeries of the locally advanced chemo-irradiated rectum tumours]. Magyar Sebészet. 2020;73(1):29-36.
- Redmond HP, Neary PM et al. RandomiSed clinical trial assessing Use of an anti-inflammatoRy aGent in attenUating peri-operatiVe inflAmmatioN in non-meTastatic colon cancer—the S.U.R.G.U.V.A.N.T. trial. BMC Cancer. 2018;18(1):794.
- Tebala GD, Gordon-Dixon A, Imtiaz M, Shrestha A, Toeima M. Enhanced recovery after rectal surgery: what we have learned so far. Mini-invasive Surgery. 2018;2:32.
- Anderson SW, Bazzell AF, Dains JE. An integrative review on the effect of prebiotics, probiotics, and synbiotics on infection after colorectal cancer surgery. AORN Journal. 2018;107(2):237–248; Aisu N, Tanimura S et al. Impact of perioperative probiotic treatment for surgical site infections in patients with colorectal cancer. Experimental and Therapeutic Medicine. 2015;10(3):966–972.
- Tebala GD, Gordon-Dixon A, Imtiaz M, Shrestha A, Toeima M. Enhanced recovery after rectal surgery: what we have learned so far. Mini-invasive Surgery. 2018;2:32.
- Tebala GD, Gordon-Dixon A, Imtiaz M, Shrestha A, Toeima M. Enhanced recovery after rectal surgery: what we have learned so far. Mini-invasive Surgery. 2018;2:32.
- Gelman D, Gelmanas A, Urbanaitė D, et al. Role of multimodal analgesia in the evolving enhanced recovery after surgery pathways. Medicina (Kaunas). 2018;54(2):20.
- Kim SY, Kim NK et al. Effects of postoperative pain management on immune function after laparoscopic resection of colorectal cancer: a randomized study [published correction appears in Medicine (Baltimore). 2016 Jun 17;95(24):e4641]. Medicine (Baltimore). 2016;95(19):e3602.
- Kim SY, Kim NK et al. Effects of postoperative pain management on immune function after laparoscopic resection of colorectal cancer: a randomized study [published correction appears in Medicine (Baltimore). 2016 Jun 17;95(24):e4641]. Medicine (Baltimore). 2016;95(19):e3602.
- Radovanović D, Radovanović Z et al. Thoracic epidural versus intravenous patient-controlled analgesia after open colorectal cancer surgery. Acta Clinica Croatia. 2017;56(2):244‐254.
- Deng W, Long X et al. Quadratus lumborum block versus transversus abdominis plane block for postoperative pain management after laparoscopic colorectal surgery: a randomized controlled trial. Medicine (Baltimore). 2019;98(52):e18448.
- Damadi AA, Lax EA, Smithson L, Pearlman RD. Comparison of therapeutic benefit of bupivacaine hcl transversus abdominis plane (TAP) block as part of an enhanced recovery pathway versus traditional oral and intravenous pain control after minimally invasive colorectal surgery: a prospective, randomized, double-blind trial. American Surgeon. 2019;85(12):1363-1368.
- Keller DS, Tahilramani RN et al. Pilot study of a novel pain management strategy: evaluating the impact on patient outcomes. Surgical Endoscopy. 2016;30(6):2192-2198.
- Pandazi A, Kapota E et al. Preincisional versus postincisional administration of parecoxib in colorectal surgery: effect on postoperative pain control and cytokine response. A randomized clinical trial. World Journal of Surgery. 2010;34(10):2463-2469.
- Yin LH, Li WS, Zhao WX, Li WY. [Role of acupuncture anesthesia in operation of rectal cancer] [Article in Chinese]. Zhongguo Zhen Jiu. 2005;25(12):876-878.
- Huang W, Yu TY, Long WF, Xiao JB. [Application of transcutaneous electrical acupoint stimulation combined with transversus abdominis plane block to enhanced recovery after surgery in patients undergoing laparoscopic colorectal cancer resection: a randomized controlled clinical trial] [Article in Chinese]. Zhen Ci Yan Jiu. 2018;43(10):611-615.
- Jamjittrong S, Matsuda A et al. Postoperative non-steroidal anti-inflammatory drugs and anastomotic leakage after gastrointestinal anastomoses: systematic review and meta-analysis. Annals of Gastroenterological Surgery. 2019;4(1):64-75; Cata JP, Guerra CE, Chang GJ, Gottumukkala V, Joshi GP. Non-steroidal anti-inflammatory drugs in the oncological surgical population: beneficial or harmful? A systematic review of the literature. British Journal of Anaesthesia. 2017 Oct 1;119(4):750-764.
- Cata JP, Guerra CE, Chang GJ, Gottumukkala V, Joshi GP. Non-steroidal anti-inflammatory drugs in the oncological surgical population: beneficial or harmful? A systematic review of the literature. British Journal of Anaesthesia. 2017;119(4):750-764.
- Liu Y, May BH et al. Acupuncture and related therapies for treatment of postoperative ileus in colorectal cancer: a systematic review and meta-analysis of randomized controlled trials. Evidence-Based Complementary and Alternative Medicine. 2018;2018:3178472; Kim KH, Kim DH, Kim HY, Son GM. Acupuncture for recovery after surgery in patients undergoing colorectal cancer resection: a systematic review and meta-analysis. Acupuncture in Medicine. 2016;34(4):248-256.
- Mai S, Meng J, Wang W, Lang S. [Influence of electroacupuncture pretreatment on intestinal function in the patients of colorectal cancer surgery] [Article in Chinese]. Zhongguo Zhen Jiu. 2017;37(5):483-487.
- Yang Y, Zuo HQ et al. Comparison of efficacy of simo decoction and acupuncture or chewing gum alone on postoperative ileus in colorectal cancer resection: a randomized trial. Scientific Reports. 2017;7:37826.
- Ng SS, Leung WW et al. Electroacupuncture reduces duration of postoperative ileus after laparoscopic surgery for colorectal cancer. Gastroenterology. 2013;144(2):307-313.e1.
- Ng SS, Leung WW et al. Electroacupuncture reduces duration of postoperative ileus after laparoscopic surgery for colorectal cancer. Gastroenterology. 2013;144(2):307-313.e1.
- Ng SS, Leung WW et al. Electroacupuncture reduces duration of postoperative ileus after laparoscopic surgery for colorectal cancer. Gastroenterology. 2013;144(2):307-313.e1.
- Wang TY, Meng JH, Mai SC. [Electroacupuncture treatment conducted before and after surgery is better in promoting reco-very of gastrointestinal function in colorectal cancer patients undergoing radical resection] [Article in Chinese]. Zhen Ci Yan Jiu. 2018;43(12):797-800.
- Guo J, Tang W et al. [Transcutaneous electrical acupoint stimulation on inflammatory response and intestinal permeability in perioperative period of laparoscopic intestinal surgery] [Article in Chinese]. Zhongguo Zhen Jiu. 2018;38(10):1043-1046.
- Huang W, Yu TY, Long WF, Xiao JB. [Application of transcutaneous electrical acupoint stimulation combined with transversus abdominis plane block to enhanced recovery after surgery in patients undergoing laparoscopic colorectal cancer resection: a randomized controlled clinical trial] [Article in Chinese]. Zhen Ci Yan Jiu. 2018;43(10):611-615.
- Tebala GD, Gordon-Dixon A, Imtiaz M, Shrestha A, Toeima M. Enhanced recovery after rectal surgery: what we have learned so far. Mini-invasive Surgery. 2018;2:32.
- Gan T, Jackson NA et al. A retrospective review: patient-reported preoperative prescription opioid, sedative, or antidepressant use is associated with worse outcomes in colorectal surgery. Diseases of the Colon & Rectum. 2020;63(7):965-973.
- Brand JM, Kirchner H, Poppe C, Schmucker P. The effects of general anesthesia on human peripheral immune cell distribution and cytokine production. Clinical Immunology and Immunopathology. 1997;83(2):190-194.
- Hiller J, Brodner G, Gottschalk A. Understanding clinical strategies that may impact tumour growth and metastatic spread at the time of cancer surgery. Best Practice & Research Clinical Anaesthesiology. 2013;27(4):427-439; Dang Y, Shi X, Xu W, Zuo M. The effect of anesthesia on the immune system in colorectal cancer patients. Gastroenterological Cancer and Immunotherapy. 2018;2018:7940603; Khanna AK, Riveros Perez E, Laudanski K, Moraska A, Cummings III KC. Perioperative care and cancer recurrence: is there a connection? World Journal of Anesthesiology. 2014 Mar 27;3(1):31-45.
- Khanna AK, Riveros Perez E, Laudanski K, Moraska A, Cummings III KC. Perioperative care and cancer recurrence: is there a connection? World Journal of Anesthesiology. 2014 Mar 27;3(1):31-45.
- Lee SK, Dawson J et al. Management of cancer pain: 1. Wider implications of orthodox analgesics. International Journal of General Medicine. 2014;7:49-58.
- Day A, Smith R et al. Retrospective analysis of the effect of postoperative analgesia on survival in patients after laparoscopic resection of colorectal cancer. British Journal of Anaesthesia. 2012;109(2):185-190.
- Kim SY, Kim NK et al. Effects of postoperative pain management on immune function after laparoscopic resection of colorectal cancer: a randomized study [published correction appears in Medicine (Baltimore). 2016 Jun 17;95(24):e4641]. Medicine (Baltimore). 2016;95(19):e3602.
- Dang Y, Shi X et al. The Effect of Anesthesia on the Immune System in Colorectal Cancer Patients. Canadian Journal of Gastroenterology & Hepatology. 2018 Apr 1;2018:7940603.
- Kim SY, Kim NK et al. Effects of Postoperative Pain Management on Immune Function After Laparoscopic Resection of Colorectal Cancer: A Randomized Study. Medicine (Baltimore). 2016 Jun 17;95(24):e4641.
- Khanna AK, Riveros Perez E, Laudanski K, Moraska A, Cummings III KC. Perioperative care and cancer recurrence: is there a connection? World Journal of Anesthesiology. 2014 Mar 27;3(1):31-45.
- Beilin B, Shavit Y et al. Effects of anesthesia based on large versus small doses of fentanyl on natural killer cell cytotoxicity in the perioperative period. Anesthesia and Analgesia. 1996 Mar;82(3):492-7.
- Biki B, Mascha E et al. Anesthetic technique for radical prostatectomy surgery affects cancer recurrence: a retrospective analysis. Anesthesiology. 2008;109(2):180-187.
- Khanna AK, Perez ER et al. Perioperative care and cancer recurrence: Is there a connection? World Journal of Anesthesiology. 2014;3(1):31-45; Dang Y, Shi X et al. The Effect of Anesthesia on the Immune System in Colorectal Cancer Patients. Canadian Journal of Gastroenterology & Hepatology. 2018 Apr 1;2018:7940603.
- Diaz-Cambronero O, Mazzinari G et al. Perioperative Opioids and Colorectal Cancer Recurrence: A Systematic Review of the Literature. Pain Management. 2018 Sep 1;8(5):353-361.
- Schack A, Fransgaard T, Klein MF, Gögenur I. Perioperative use of nonsteroidal anti-inflammatory drugs decreases the risk of recurrence of cancer after colorectal resection: a cohort study based on prospective data. Annals of Surgical Oncology. 2019;26(12):3826-3837.
- Lönnroth C, Andersson M et al. Preoperative treatment with a non-steroidal anti-inflammatory drug (NSAID) increases tumor tissue infiltration of seemingly activated immune cells in colorectal cancer. Cancer Immunity. 2008;8:5.
- Schack A, Fransgaard T, Klein MF, Gögenur I. Perioperative use of nonsteroidal anti-inflammatory drugs decreases the risk of recurrence of cancer after colorectal resection: a cohort study based on prospective data. Annals of Surgical Oncology. 2019 Nov;26(12):3826-3837.
- Cata JP, Guerra CE, Chang GJ, Gottumukkala V, Joshi GP. Non-steroidal anti-inflammatory drugs in the oncological surgical population: beneficial or harmful? A systematic review of the literature. British Journal of Anaesthesia. 2017 Oct 1;119(4):750-764.
- Dang Y, Shi X et al. The effect of anesthesia on the immune system in colorectal cancer patients. Canadian Journal of Gastroenterology & Hepatology. 2018 Apr 1;2018:7940603.
- Panigrahy D, Gartung A et al. Preoperative stimulation of resolution and inflammation blockade eradicates micrometastases. Journal of Clinical Investigation. 2019;129(7):2964-2979.
- Cata JP, Guerra CE, Chang GJ, Gottumukkala V, Joshi GP. Non-steroidal anti-inflammatory drugs in the oncological surgical population: beneficial or harmful? A systematic review of the literature. British Journal of Anaesthesia. 2017 Oct 1;119(4):750-764.
- Jia N, Sun Z et al. Serial monitoring of circulating tumor DNA in patients with metastatic colorectal cancer to predict the therapeutic response. Frontiers in Genetics. 2019 May 21;10:470.
- Reece M, Saluja H et al. The use of circulating tumor DNA to monitor and predict response to treatment in colorectal cancer. Frontiers in Genetics. 2019;10:1118.
- Tie J, Cohen JD et al. Circulating tumor DNA analyses as markers of recurrence risk and benefit of adjuvant therapy for stage III colon cancer [published correction Error in Figure 2D appears in JAMA Oncology. 2019 Dec 1;5(12):1811]. JAMA Oncology. 2019;5(12):1710-1717.
- Baek DH, Kim GH et al. Clinical potential of circulating tumor cells in colorectal cancer: a prospective study. Clinical and Translational Gastroenterology. 2019;10(7):e00055.
- Yang C, Chen F, Wang S, Xiong B. Circulating tumor cells in gastrointestinal cancers: current status and future perspectives. Frontiers in Oncology. 2019;9:1427; Burz C, Pop VV et al. Circulating tumor cells in clinical research and monitoring patients with colorectal cancer. Oncotarget. 2018;9(36):24561-24571.
- Alschuler LN, Gazella KA. The Definitive Guide to Thriving after Cancer: A Five-Step Integrative Plan to Reduce the Risk of Recurrence and Build Lifelong Health. Berkeley, California: Ten Speed Press. 2013.
- Cancer Treatment Centers of America. Colorectal cancer types. September 15, 2020. Viewed September 16, 2020.
- CancerNet. Colorectal Cancer: Introduction. October 2019. Viewed September 16, 2020.
- American Cancer Society. Colorectal Cancer Signs and Symptoms. June 29, 2020. Viewed September 16, 2020.
- Centers for Disease Control and Prevention. Basic Information About Colorectal Cancer. US Department of Health & Human Services. February 10, 2020. Viewed September 16, 2020.
- American Cancer Society. Colorectal Cancer Facts & Figures 2020-2022. Viewed November 11, 2020.
- Siegel RL, Fedewa SA et al. Colorectal cancer incidence patterns in the United States, 1974-2013. Journal of the National Cancer Institute. 2017 Aug 1;109(8).
- Arhi CS, Ziprin P et al. Colorectal cancer patients under the age of 50 experience delays in primary care leading to emergency diagnoses: a population-based study. Colorectal Disease. 2019 Nov;21(11):1270-1278.
- Baak JP, Gyllenhaal C, Liu L, Guo H, Block KI. Prognostic proof and possible therapeutic mechanisms of herbal medicine in patients with metastatic lung and colon cancer. Integrative Cancer Therapies. 2011 Sep;10(3):NP1-NP11.
- Block KI, Block PB, Gyllenhaal C. Integrative treatment for colorectal cancer: a comprehensive approach. The Journal of Alternative And Complementary Medicine. 2018 Sep/Oct;24(9-10):890-901.
- Steele SR, Chang GJ et al. Practice guideline for the surveillance of patients after curative treatment of colon and rectal cancer. Diseases of the Colon and Rectum. 2015 Aug;58(8):713-25.
- Bibbins-Domingo K; US Preventive Services Task Force. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: US Preventive Services Task Force recommendation statement. Annals of Internal Medicine. 2016 Jun 21;164(12):836-45.
- Leanna Standish. Email communication with Laura Pole. September 28, 2018.
- Integrative oncology study draws attention for promising results. Bastyr University News. December 4, 2013 Viewed May 17, 2019.
- Survival Rates for Colorectal Cancer. American Cancer Society. June 29, 2020. Viewed November 16, 2020.
- Block KI, Block PB, Gyllenhaal C. Integrative treatment for colorectal cancer: a comprehensive approach. Journal of Alternative and Complementary Medicine. 2018 Sep/Oct;24(9-10):890-901.
- Alschuler LN, Gazella KA. The Definitive Guide to Cancer, 3rd Edition: An Integrative Approach to Prevention, Treatment, and Healing. Berkeley, California: Celestial Arts. 2010; Alschuler LN, Gazella KA. The Definitive Guide to Thriving after Cancer: A Five-Step Integrative Plan to Reduce the Risk of Recurrence and Build Lifelong Health. Berkeley, California: Ten Speed Press. 2013.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Cohen L, Jefferies A. Anticancer Living: Transform Your Life and Health with the Mix of Six. New York: Viking. 2018.
- Lemole G, Mehta P, McKee D. After Cancer Care: The Definitive Self-Care Guide to Getting and Staying Well for Patients with Cancer. New York, New York: Rodale, Inc. 2015.
- McKinney N. Naturopathic Oncology, 3rd Edition. Victoria, BC, Canada: Liaison Press. 2016.
- Parmar G, Kaczor T. Textbook of Naturopathic Oncology: A Desktop Guide of Integrative Cancer Care. 1st edition. Canada: Medicatrix Holdings Ltd. 2020.
- Chan KK, Yao TJ et al. The use of Chinese herbal medicine to improve quality of life in women undergoing chemotherapy for ovarian cancer: a double-blind placebo-controlled randomized trial with immunological monitoring. Annals of Oncology. 2011 Oct;22(10):2241-9.
- Bae K, Kim E, Choi JJ, Kim MK, Yoo HS. The effectiveness of anticancer traditional Korean medicine treatment on the survival in patients with lung, breast, gastric, colorectal, hepatic, uterine, or ovarian cancer: a prospective cohort study protocol. Medicine (Baltimore). 2018 Oct;97(41):e12444.
- Lemole G, Mehta P, McKee D. After Cancer Care: The Definitive Self-Care Guide to Getting and Staying Well for Patients with Cancer. New York, New York: Rodale, Inc. 2015.
- Romaguera D, Ward H et al. Pre-diagnostic concordance with the WCRF/AICR guidelines and survival in European colorectal cancer patients: a cohort study. BMC Med. 2015 May 7;13:107.
- Van Blarigan EL, Fuchs CS et al. Association of survival with adherence to the American Cancer Society Nutrition and Physical Activity Guidelines for Cancer Survivors after colon cancer diagnosis: The CALGB 89803/Alliance Trial. JAMA Oncology. 2018 Apr 12.
- Matsell SL, Sánchez-García MA, Halliday V, Williams EA, Corfe BM. Investigating the nutritional advice and support given to colorectal cancer survivors in the UK: is it fit for purpose and does it address their needs? Journal of Human Nutrition and Dietetics. 2020 Sep 20.
- Song M, Wu K et al. Low-carbohydrate diet score and macronutrient intake in relation to survival after colorectal cancer diagnosis. JNCI Cancer Spectrum. 2018 Nov;2(4):pky077.
- Volpato M, Hull MA. Omega-3 polyunsaturated fatty acids as adjuvant therapy of colorectal cancer. Cancer Metastasis Reviews. 2018 Sep;37(2-3):545-555; Song M, Ou FS et al. Marine omega-3 fatty acid intake and survival of stage III colon cancer according to tumor molecular markers in NCCTG Phase III trial N0147 (Alliance). International Journal of Cancer. 2019 Jul 15;145(2):380-389; Song M, Zhang X et al. Marine ω-3 polyunsaturated fatty acid intake and survival after colorectal cancer diagnosis. Gut. 2017 Oct;66(10):1790-1796.
- Morales-Oyarvide V, Yuan C et al. Dietary insulin load and cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803 (Alliance). Journal of the National Cancer Institute. 2019 Feb 1;111(2):170-179; Keum N, Yuan C et al. Dietary glycemic and insulin scores and colorectal cancer survival by tumor molecular biomarkers. International Journal of Cancer. 2017 Jun 15;140(12):2648-2656.
- Lee J, Jeon JY, Meyerhardt JA. Diet and lifestyle in survivors of colorectal cancer. Hematology/Oncology Clinics of North America. 2015 Feb;29(1):1-27; Meyerhardt JA, Niedzwiecki D et al. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. JAMA. 2007 Aug 15;298(7):754-64.
- Lee J, Jeon JY, Meyerhardt JA. Diet and lifestyle in survivors of colorectal cancer. Hematology/Oncology Clinics of North America. 2015 Feb;29(1):1-27.
- Abrams DI, Weil AT. Chapter 7, Botanical and Mycological Medicine in Integrative Oncology Integrative Oncology, 2nd Edition. New York, NY: Oxford University Press. 2014.
- Fadelu T, Zhang S et al. Nut consumption and survival in patients with stage iii colon cancer: results from CALGB 89803 (Alliance). Journal of Clinical Oncology. 2018 Feb 28:JCO2017755413.
- Zheng J, Tabung FK et al. Post-cancer diagnosis dietary inflammatory potential is associated with survival among women diagnosed with colorectal cancer in the Women's Health Initiative. European Journal of Nutrition. 2019 Apr 6.
- Wesselink E, Winkels RM et al. Dietary intake of magnesium or calcium and chemotherapy-induced peripheral neuropathy in colorectal cancer patients. Nutrients. 2018 Mar 23;10(4). pii: E398.
- Cleveland Clinic. Magnesium Rich Food. December 1, 2014. Viewed September 16, 2020.
- CancerNet. Peripheral Neuropathy. American Society of Clinical Oncology. May 2017. Viewed March 2, 2020.
- McCulloch M. 15 Healthy Foods High in B Vitamins. Healthline. October 11, 2018. Viewed September 21, 2020.
- Zhu Y, Wu H et al. Dietary patterns and colorectal cancer recurrence and survival: a cohort study. BMJ Open. 2013 Feb 7;3(2). pii: e002270; Meyerhardt JA, Niedzwiecki D et al. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. Journal of the American Medical Association. 2007 Aug 15;298(7):754-64.
- Zhang FF, Cudhea F et al. Preventable cancer burden associated with poor diet in the United States. JNCI Cancer Spectrum. 2019 May;pkz034.
- American Institute for Cancer Research. Colorectal Cancer: Arm yourself with information. December 18, 2019. Viewed September 18, 2020.
- Steele SR, Chang GJ et al. Practice guideline for the surveillance of patients after curative treatment of colon and rectal cancer. Diseases of the Colon and Rectum. 2015 Aug;58(8):713-25.
- Farinetti A, Zurlo V, Manenti A, Coppi F, Mattioli AV. Mediterranean diet and colorectal cancer: a systematic review. Nutrition. 2017 Nov - Dec;43-44:83-88.
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Diet, nutrition, physical activity and colorectal cancer. Viewed May 14, 2019; Thanikachalam K, Khan G. Colorectal cancer and nutrition. Nutrients. 2019 Jan 14;11(1). pii: E164; Meyerhardt JA, Niedzwiecki D et al. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. JAMA. 2007 Aug 15;298(7):754-64; Song M, Chan AT. Environmental factors, gut microbiota, and colorectal cancer prevention. Clinical Gastroenterology and Hepatology. 2019 Jan;17(2):275-289; Murphy N, Norat T et al. Consumption of dairy products and colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC). PLoS One. 2013 Sep 2;8(9):e72715; Larsson SC, Bergkvist L, Wolk A. High-fat dairy food and conjugated linoleic acid intakes in relation to colorectal cancer incidence in the Swedish Mammography Cohort. American Journal of Clinical Nutrition. 2005 Oct;82(4):894-900; Bailie L, Loughrey MB, Coleman HG. Lifestyle risk factors for serrated colorectal polyps: a systematic review and meta-analysis. Gastroenterology. 2017 Jan;152(1):92-104; Kronborg O.Endoscopy. Colon polyps and cancer. 2004 Jan;36(1):3-7; Katona BW, Weiss JM. Chemoprevention of colorectal cancer. Gastroenterology. 2020;158(2):368–388; Chapelle N, Martel M, Toes-Zoutendijk E, Barkun AN, Bardou M. Recent advances in clinical practice: colorectal cancer chemoprevention in the average-risk population. Gut. 2020 Sep 28:gutjnl-2020-320990; Veettil SK, Wong TY et al. Role of diet in colorectal cancer incidence: umbrella review of meta-analyses of prospective observational studies. JAMA Network Open. 2021 Feb 1;4(2):e2037341.
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Diet, nutrition, physical activity and colorectal cancer. Viewed May 14, 2019; Bradbury KE, Murphy N, Key TJ. Diet and colorectal cancer in UK Biobank: a prospective study. International Journal of Epidemiology. 2019 Apr 17. pii: dyz064; Meyerhardt JA, Niedzwiecki D et al. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. JAMA. 2007 Aug 15;298(7):754-64; Bailie L, Loughrey MB, Coleman HG. Lifestyle risk factors for serrated colorectal polyps: a systematic review and meta-analysis. Gastroenterology. 2017 Jan;152(1):92-104; Dolejs SC, Gayed B, Fajardo A. Prevention of colorectal neoplasia. Clinics in Colon and Rectal Surgery. 2016;29(4):353-362; Chapelle N, Martel M, Toes-Zoutendijk E, Barkun AN, Bardou M. Recent advances in clinical practice: colorectal cancer chemoprevention in the average-risk population. Gut. 2020 Sep 28:gutjnl-2020-320990; Veettil SK, Wong TY et al. Role of diet in colorectal cancer incidence: umbrella review of meta-analyses of prospective observational studies. JAMA Network Open. 2021 Feb 1;4(2):e2037341.
- Thanikachalam K, Khan G. Colorectal cancer and nutrition. Nutrients. 2019 Jan 14;11(1). pii: E164; Bultman SJ. Emerging roles of the microbiome in cancer. Carcinogenesis. 2014;35(2):249–255.
- Katona BW, Weiss JM. Chemoprevention of colorectal cancer. Gastroenterology. 2020;158(2):368–388.
- Beresford SA, Johnson KC et al. Low-fat dietary pattern and risk of colorectal cancer: the Women's Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006 Feb 8;295(6):643-54.
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Diet, nutrition, physical activity and colorectal cancer. Viewed May 14, 2019; Durko L, Malecka-Panas E et al. Lifestyle modifications and colorectal cancer. Current Colorectal Cancer Reports. 2014;10:45-54; Thanikachalam K, Khan G. Colorectal cancer and nutrition. Nutrients. 2019 Jan 14;11(1). pii: E164; Durko L, Malecka-Panas E et al. Lifestyle modifications and colorectal cancer. Current Colorectal Cancer Reports. 2014;10:45-54; Thanikachalam K, Khan G. Colorectal cancer and nutrition. Nutrients. 2019 Jan 14;11(1). pii: E164; Shivappa N, Godos J et al. Dietary inflammatory index and colorectal cancer risk-a meta-analysis. Nutrients. 2017 Sep 20;9(9). pii: E1043; Tabung FK, Liu L et al. Association of dietary inflammatory potential with colorectal cancer risk in men and women. JAMA Oncology. 2018 Mar 1;4(3):366-373; Bailie L, Loughrey MB, Coleman HG. Lifestyle risk factors for serrated colorectal polyps: a systematic review and meta-analysis. Gastroenterology. 2017 Jan;152(1):92-104; Green CJ, de Dauwe P et al. Tea, coffee, and milk consumption and colorectal cancer risk. Journal of Epidemiology. 2014;24(2):146-53; Su LJ, Arab L. Tea consumption and the reduced risk of colon cancer—results from a national prospective cohort study. Public Health Nutrition. 2002 Jun;5(3):419-25; Wada K, Oba S et al. Green tea intake and colorectal cancer risk in Japan: the Takayama study. Japanese Journal of Clinical Oncology. 2019 Jun 1;49(6):515-520; Ullah MF, Bhat SH et al. Pharmacological intervention through dietary nutraceuticals in gastrointestinal neoplasia. Critical Reviews in Food Science and Nutrition. 2016 Jul 3;56(9):1501-18; Arab L, Il'yasova D. The epidemiology of tea consumption and colorectal cancer incidence. Journal of Nutrition. 2003 Oct;133(10):3310S-3318S; Chen Y, Wu Y et al. An inverse association between tea consumption and colorectal cancer risk. Oncotarget. 2017 Jun 6;8(23):37367-37376; Sun CL, Yuan JM, Koh WP, Yu MC. Green tea, black tea and colorectal cancer risk: a meta-analysis of epidemiologic studies. Carcinogenesis. 2006 Jul;27(7):1301-9; Mackintosh C, Yuan C et al. Association of coffee intake with survival in patients with advanced or metastatic colorectal cancer. JAMA Oncology. 2020;10.1001/jamaoncol.2020.3938; Ngo SN, Williams DB, Cobiac L, Head RJ. Does garlic reduce risk of colorectal cancer? A systematic review. Journal of Nutrition. 2007 Oct;137(10):2264-9; Fleischauer AT, Poole C, Arab L. Garlic consumption and cancer prevention: meta-analyses of colorectal and stomach cancers. American Journal of Clinical Nutrition. 2000 Oct;72(4):1047-52; Bobe G, Sansbury LB et al. Dietary flavonoids and colorectal adenoma recurrence in the Polyp Prevention Trial. Cancer Epidemiology, Biomarkers & Prevention. 2008;17(6):1344-1353; Chang H, Lei L, Zhou Y, Ye F, Zhao G. Dietary flavonoids and the risk of colorectal cancer: an updated meta-analysis of epidemiological studies. Nutrients. 2018;10(7):950; Zheng X, Wu K et al. Yogurt consumption and risk of conventional and serrated precursors of colorectal cancer. Gut. 2019;gutjnl-2019-318374; Chapelle N, Martel M, Toes-Zoutendijk E, Barkun AN, Bardou M. Recent advances in clinical practice: colorectal cancer chemoprevention in the average-risk population. Gut. 2020 Sep 28:gutjnl-2020-320990; Touvier M, Chan DS et alT. Meta-analyses of vitamin D intake, 25-hydroxyvitamin D status, vitamin D receptor polymorphisms, and colorectal cancer risk. Cancer Epidemiology, Biomarkers & Prevention. 2011 May;20(5):1003-16; ; Veettil SK, Wong TY et al. Role of diet in colorectal cancer incidence: umbrella review of meta-analyses of prospective observational studies. JAMA Network Open. 2021 Feb 1;4(2):e2037341; Kim DH, Smith-Warner SA et al. Pooled analyses of 13 prospective cohort studies on folate intake and colon cancer. Cancer Causes & Control. 2010 Nov;21(11):1919-30; Gibson TM, Weinstein SJ et al. Pre- and postfortification intake of folate and risk of colorectal cancer in a large prospective cohort study in the United States. American Journal of Clinical Nutrition. 2011 Oct;94(4):1053-62; Giovannucci E. Epidemiologic studies of folate and colorectal neoplasia: a review. Journal of Nutrition. 2002 Aug;132(8 Suppl):2350S-2355S.
- Fleischauer AT, Arab L. Garlic and cancer: a critical review of the epidemiologic literature. Journal of Nutrition. 2001 Mar;131(3s):1032S-40S; Fleischauer AT, Poole C, Arab L. Garlic consumption and cancer prevention: meta-analyses of colorectal and stomach cancers. American Journal of Clinical Nutrition. 2000 Oct;72(4):1047-52.
- Chiavarini M, Minelli L, Fabiani R. Garlic consumption and colorectal cancer risk in man: a systematic review and meta-analysis. Public Health Nutrition. 2016 Feb;19(2):308-17; Baena R, Salinas P. Diet and colorectal cancer. Maturitas. 2015 Mar;80(3):258-64; Hu JY, Hu YW et al. Consumption of garlic and risk of colorectal cancer: an updated meta-analysis of prospective studies. World Journal of Gastroenterology. 2014 Nov 7;20(41):15413-22; Chapelle N, Martel M, Toes-Zoutendijk E, Barkun AN, Bardou M. Recent advances in clinical practice: colorectal cancer chemoprevention in the average-risk population. Gut. 2020 Sep 28:gutjnl-2020-320990; Wan Q, Li N et al. Allium vegetable consumption and health: an umbrella review of meta-analyses of multiple health outcomes. Food Science & Nutrition. 2019 Jul 10;7(8):2451-2470.
- Chapelle N, Martel M, Toes-Zoutendijk E, Barkun AN, Bardou M. Recent advances in clinical practice: colorectal cancer chemoprevention in the average-risk population. Gut. 2020 Sep 28:gutjnl-2020-320990.
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Diet, nutrition, physical activity and colorectal cancer. Viewed October 19, 2020.
- Thanikachalam K, Khan G. Colorectal cancer and nutrition. Nutrients. 2019 Jan 14;11(1). pii: E164; Foods that fight inflammation. Harvard Health Publishing. August 29, 2020. Viewed October 19, 2020.
- Dolejs SC, Gayed B, Fajardo A. Prevention of colorectal neoplasia. Clinics in Colon and Rectal Surgery. 2016;29(4):353-362.
- Zhang SM, Moore SC et al. Folate, vitamin B6, multivitamin supplements, and colorectal cancer risk in women. American Journal of Epidemiology. 2006;163(2):108–115; de Vogel S, Dindore V et al. Dietary folate, methionine, riboflavin, and vitamin B-6 and risk of sporadic colorectal cancer. Journal of Nutrition. 2008;138(12):2372–2378; Ben S, Du M et al. Vitamin B2 intake reduces the risk for colorectal cancer: a dose-response analysis. European Journal of Nutrition. 2019;58(4):1591–1602.
- Schernhammer ES, Giovannuccci E, Fuchs CS, Ogino S. A prospective study of dietary folate and vitamin B and colon cancer according to microsatellite instability and KRAS mutational status. Cancer Epidemiology, Biomarkers & Prevention. 2008;17(10):2895–2898.
- Banjari I, Kožić S. Dietary intake of vitamin B12 in relation to diet and lifestyle characteristics in a population at high risk for colorectal cancer. Central European Journal of Public Health. 2018;26(4):253–259.
- National Health Service. B vitamins and folic acid. August 20, 2020. Viewed September 16, 2020.
- Zhang SM, Moore SC et al. Folate, vitamin B6, multivitamin supplements, and colorectal cancer risk in women. American Journal of Epidemiology. 2006;163(2):108–115; Schernhammer ES, Giovannuccci E, Fuchs CS, Ogino S. A prospective study of dietary folate and vitamin B and colon cancer according to microsatellite instability and KRAS mutational status. Cancer Epidemiology, Biomarkers & Prevention. 2008;17(10):2895–2898.
- Weinstein SJ, Albanes D, Selhub J, et al. One-carbon metabolism biomarkers and risk of colon and rectal cancers. Cancer Epidemiology, Biomarkers and Prevention. 2008;17(11):3233–3240.
- Jia K, Wang R, Tian J. Vitamin B6 intake and the risk of colorectal cancer: a meta-analysis of prospective cohort studies. Nutrition and Cancer. 2017;69(5):723–731.
- de Vogel S, Dindore V et al. Dietary folate, methionine, riboflavin, and vitamin B-6 and risk of sporadic colorectal cancer. Journal of Nutrition. 2008;138(12):2372–2378.
- Zhang SM, Moore SC et al. Folate, vitamin B6, multivitamin supplements, and colorectal cancer risk in women. American Journal of Epidemiology. 2006;163(2):108–115.
- Yang CY, Chiu HF, Chiu JF, Tsai SS, Cheng MF. Calcium and magnesium in drinking water and risk of death from colon cancer. Japanese Journal of Cancer Research. 1997 Oct;88(10):928-33.
- Chen GC, Pang Z, Liu QF. Magnesium intake and risk of colorectal cancer: a meta-analysis of prospective studies. European Journal of Clinical Nutrition. 2012 Nov;66(11):1182-6; Crosara Teixeira M, Braghiroli MI, Sabbaga J, Hoff PM. Primary prevention of colorectal cancer: myth or reality? World Journal of Gastroenterology. 2014 Nov 7;20(41):15060-9; Thanikachalam K, Khan G. Colorectal cancer and nutrition. Nutrients. 2019 Jan 14;11(1). pii: E164.
- Zhu X, Shrubsole MJ et al. Calcium/magnesium intake ratio, but not magnesium intake, interacts with genetic polymorphism in relation to colorectal neoplasia in a two-phase study. Molecular Carcinogenesis. 2016 Oct;55(10):1449-57; Bailie L, Loughrey MB, Coleman HG. Lifestyle risk factors for serrated colorectal polyps: a systematic review and meta-analysis. Gastroenterology. 2017 Jan;152(1):92-104; Song M, Chan AT. Environmental factors, gut microbiota, and colorectal cancer prevention. Clinical Gastroenterology and Hepatology. 2019 Jan;17(2):275-289.
- Orchel A, Dzierzewicz Z, Parfiniewicz B, Weglarz L, Wilczok T. Butyrate-induced differentiation of colon cancer cells is PKC and JNK dependent. Dig Dis Sci. 2005 Mar;50(3):490-8.
- Bultman SJ. Emerging roles of the microbiome in cancer. Carcinogenesis. 2014;35(2):249–255.
- Liu K, Zhou R et al. Effect of green tea on glucose control and insulin sensitivity: a meta-analysis of 17 randomized controlled trials. American Journal of Clinical Nutrition. 2013 Aug;98(2):340-8.
- Martín MA, Goya L, Ramos S. Preventive effects of cocoa and cocoa antioxidants in colon cancer. Diseases. 2016;4(1):6.
- Qu B, Qu H. The influence of statins on risk and patient survival in colorectal cancer. Journal of Clinical Gastroenterology. 2019;53(9):699–701.
- Meyerhardt JA, Giovannucci EL et al. Physical activity and survival after colorectal cancer diagnosis. Journal of Clinical Oncology. 2006 Aug 1;24(22):3527-34; Lee J, Jeon JY, Meyerhardt JA. Diet and lifestyle in survivors of colorectal cancer. Hematology/Oncology Clinics of North America. 2015 Feb;29(1):1-27; Li T, Wei S et al. The dose-response effect of physical activity on cancer mortality: findings from 71 prospective cohort studies. British Journal of Sports Medicine. 2016 Mar;50(6):339-45; Ratjen I, Schafmayer C et al. Postdiagnostic physical activity, sleep duration, and TV watching and all-cause mortality among long-term colorectal cancer survivors: a prospective cohort study. BMC Cancer. 2017 Oct 25;17(1):701.
- Marshall CH, Al-Mallah MH et al. Cardiorespiratory fitness and incident lung and colorectal cancer in men and women: results from the Henry Ford Exercise Testing (FIT) cohort. Cancer. 2019 May 6.
- Van Blarigan EL, Meyerhardt JA. Role of physical activity and diet after colorectal cancer diagnosis. Journal of Clinical Oncology. 2015 Jun 1;33(16):1825-34.
- Harvard Women's Health Watch. MET-hour equivalents of various physical activities. Harvard Health Publishing. December 2009. Viewed February 27, 2019.
- Blanchard CM, Courneya KS, Stein K; American Cancer Society's SCS-II. Cancer survivors' adherence to lifestyle behavior recommendations and associations with health-related quality of life: results from the American Cancer Society's SCS-II. Journal of Clinical Oncology. 2008 May 1;26(13):2198-204.
- Johnson BL, Trentham-Dietz A, Koltyn KF, Colbert LH. Physical activity and function in older, long-term colorectal cancer survivors. Cancer Causes & Control. 2009 Jul;20(5):775-84.
- Lynch BM, Cerin E, Owen N, Aitken JF. Associations of leisure-time physical activity with quality of life in a large, population-based sample of colorectal cancer survivors. Cancer Causes & Control. 2007 Sep;18(7):735-42; Courneya KS, Friedenreich CM. Physical exercise and quality of life following cancer diagnosis: a literature review. Annals of Behavioral Medicine. 1999 Spring;21(2):171-9.
- Peddle CJ, Au HJ, Courneya KS. Associations between exercise, quality of life, and fatigue in colorectal cancer survivors. Diseases of the Colon and Rectum. 2008 Aug;51(8):1242-8.
- Courneya KS, Friedenreich CM et al. A randomized trial of exercise and quality of life in colorectal cancer survivors. European Journal of Cancer Care (Engl). 2003 Dec;12(4):347-57.
- Courneya KS, Friedenreich CM. Relationship between exercise pattern across the cancer experience and current quality of life in colorectal cancer survivors. Journal of Alternative and Complementary Medicine. 1997 Fall;3(3):215-26.
- Peddle CJ, Au HJ, Courneya KS. Associations between exercise, quality of life, and fatigue in colorectal cancer survivors. Diseases of the Colon and Rectum. 2008 Aug;51(8):1242-8.
- Courneya KS, Friedenreich CM. Physical exercise and quality of life following cancer diagnosis: a literature review. Annals of Behavioral Medicine. 1999 Spring;21(2):171-9.
- Courneya KS, Friedenreich CM. Physical exercise and quality of life following cancer diagnosis: a literature review. Annals of Behavioral Medicine. 1999 Spring;21(2):171-9.
- Exercising for Better Sleep. Johns Hopkins Medicine. Viewed November 25, 2020.
- Coles T, Bennett AV et al. Relationship between sleep and exercise as colorectal cancer survivors transition off treatment. Supportive Care in Cancer. 2018 Aug;26(8):2663-2673.
- Steele SR, Chang GJ et al. Practice guideline for the surveillance of patients after curative treatment of colon and rectal cancer. Diseases of the Colon and Rectum. 2015 Aug;58(8):713-25.
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Diet, nutrition, physical activity and colorectal cancer. Viewed May 14, 2019; Samad AK, Taylor RS, Marshall T, Chapman MA. A meta-analysis of the association of physical activity with reduced risk of colorectal cancer. Colorectal Disease. 2005 May;7(3):204-13; Wolin KY, Yan Y, Colditz GA, Lee IM. Physical activity and colon cancer prevention: a meta-analysis. British Journal of Cancer. 2009 Feb 24;100(4):611-6; Dolejs SC, Gayed B, Fajardo A. Prevention of colorectal neoplasia. Clinics in Colon and Rectal Surgery. 2016;29(4):353-362.
- Marshall CH, Al-Mallah MH et al. Cardiorespiratory fitness and incident lung and colorectal cancer in men and women: results from the Henry Ford Exercise Testing (FIT) cohort. Cancer. 2019 May 6; Crosara Teixeira M, Braghiroli MI, Sabbaga J, Hoff PM. Primary prevention of colorectal cancer: myth or reality? World Journal of Gastroenterology. 2014 Nov 7;20(41):15060-9; Song M, Chan AT. Environmental factors, gut microbiota, and colorectal cancer prevention. Clinical Gastroenterology and Hepatology. 2019 Jan;17(2):275-289.
- Matthews CE, Moore SC et al. Amount and intensity of leisure-time physical activity and lower cancer risk. Journal of Clinical Oncology. 2019 Dec 26:JCO1902407.
- Kikuchi N, Nishiyama T et al. Perceived stress and colorectal cancer incidence: the Japan Collaborative Cohort Study. Scientific Reports. 2017 Jan 16;7:40363.
- Xiao Q, Arem H, Pfeiffer R, Matthews C. Prediagnosis sleep duration, napping, and mortality among colorectal cancer survivors in a large US cohort. Sleep. 2017 Apr 1;40(4).
- Xiao Q, Arem H, Pfeiffer R, Matthews C. Prediagnosis sleep duration, napping, and mortality among colorectal cancer survivors in a large US cohort. Sleep. 2017 Apr 1;40(4).
- Ratjen I, Schafmayer C et al. Postdiagnostic physical activity, sleep duration, and TV watching and all-cause mortality among long-term colorectal cancer survivors: a prospective cohort study. BMC Cancer. 2017 Oct 25;17(1):701.
- Mormont MC, Waterhouse J et al. Marked 24-h rest/activity rhythms are associated with better quality of life, better response, and longer survival in patients with metastatic colorectal cancer and good performance status. Clinical Cancer Research. 2000 Aug;6(8):3038-45.
- Innominato PF, Giacchetti S et al. Prediction of overall survival through circadian rest-activity monitoring during chemotherapy for metastatic colorectal cancer. International Journal of Cancer. 2012 Dec 1;131(11):2684-92.
- Innominato PF, Giacchetti S et al. Fatigue and weight loss predict survival on circadian chemotherapy for metastatic colorectal cancer. Cancer. 2013 Jul 15;119(14):2564-73.
- Mormont MC, Waterhouse J et al. Marked 24-h rest/activity rhythms are associated with better quality of life, better response, and longer survival in patients with metastatic colorectal cancer and good performance status. Clinical Cancer Research. 2000 Aug;6(8):3038-45; Stouffer J, Block PB et al. Correlations between circadian rhythms and quality of life in cancer patients. Presented at the annual meeting of the Society for Integrative Oncology, November 2006, Boston Massachusetts.
- Coles T, Bennett AV et al. Relationship between sleep and exercise as colorectal cancer survivors transition off treatment. Supportive Care Cancer. 2018 Aug;26(8):2663-2673.
- Steele SR, Chang GJ et al. Practice guideline for the surveillance of patients after curative treatment of colon and rectal cancer. Diseases of the Colon and Rectum. 2015 Aug;58(8):713-25.
- Durko L, Malecka-Panas E et al. Lifestyle modifications and colorectal cancer. Current Colorectal Cancer Reports. 2014;10:45-54.
- Zhao H, Yin JY et al. Sleep duration and cancer risk: a systematic review and meta-analysis of prospective studies. Asian Pacific Journal of Cancer Prevention. 2013;14(12):7509-15.
- Dun A, Zhao X et al. Association between night-shift work and cancer risk: updated systematic review and meta-analysis. Frontiers in Oncology. 2020 Jun 23;10:1006.
- Block KI, Block PB, Gyllenhaal C. Integrative treatment for colorectal cancer: a comprehensive approach. Journal of Alternative and Complementary Medicine. 2018 Sep/Oct;24(9-10):890-901.
- Janssen S, Solomon G, SchettlerT. Toxicant and Disease Database. Collaborative on Health and the Environment. 2011. Viewed May 20, 2019; Ward MH, Jones RR. Drinking water nitrate and human health: an updated review. International Journal of Environmental Research and Public Health. 2018 Jul 23;15(7). pii: E1557.
- Nogacka AM, Gómez-Martín M et al. Xenobiotics formed during food processing: their relation with the intestinal microbiota and colorectal cancer. International Journal of Molecular Sciences. 2019 Apr 25;20(8). pii: E2051.
- Kotronoulas G, Papadopoulou C, Burns-Cunningham K, Simpson M, Maguire R. A systematic review of the supportive care needs of people living with and beyond cancer of the colon and/or rectum. European Journal of Oncology Nursing. 2017 Aug;29:60-70.
- Goldzweig G, Hubert A et al. Gender and psychological distress among middle- and older-aged colorectal cancer patients and their spouses: an unexpected outcome. Critical Reviews in Oncology/Hematology. 2009 Apr;70(1):71-82.
- Gonzalez-Saenz de Tejada M, Bilbao A et al. Association between social support, functional status, and change in health-related quality of life and changes in anxiety and depression in colorectal cancer patients. Psychooncology. 2017 Sep;26(9):1263-1269.
- Dong X, Li G2 et al. The mediating role of resilience in the relationship between social support and posttraumatic growth among colorectal cancer survivors with permanent intestinal ostomies: A structural equation model analysis. European Journal of Oncology Nursing. 2017 Aug;29:47-52.
- Haviland J, Sodergren S et al. Social support following diagnosis and treatment for colorectal cancer and associations with health-related quality of life: results from the UK ColoREctal Wellbeing (CREW) cohort study. Psychooncology. 2017 Dec;26(12):2276-2284.
- Creagan ET. Psychosocial issues in oncologic practice. Mayo Clinic Proceedings. 1993 Feb;68(2):161-7.
- Rogers CR, Mitchell JA, Franta GJ, Foster MJ, Shires D. Masculinity, racism, social support, and colorectal cancer screening uptake among African American men: a systematic review. American Journal of Men’s Health. 2017 Sep;11(5):1486-1500.
- Dunn J, Lynch B et al. Dimensions of quality of life and psychosocial variables most salient to colorectal cancer patients. Psychooncology. 2006 Jan;15(1):20-30.
- Davenport L. Drug-drug interactions to avoid in patients with GI cancer. Medscape Oncology. July 8, 2020. Viewed July 13, 2020.
- Alyami M, Hübner M et al. Pressurised intraperitoneal aerosol chemotherapy: rationale, evidence, and potential indications. Lancet Oncology. 2019 Jul;20(7):e368-e377.
- Pulsed low-dose pelvic reirradiation safe, provided tangible benefit for patients with few other options. Fox Chase Cancer Center, September 22, 2020. Viewed September 30, 2020; Paly JJ, Deng M et al. Pelvic reirradiation utilizing pulsed low-dose rate radiation therapy. American Journal of Clinical Oncology. 2020 Oct;43(10):748-751.
- Baak JP, Gyllenhaal C, Liu L, Guo H, Block KI. Prognostic proof and possible therapeutic mechanisms of herbal medicine in patients with metastatic lung and colon cancer. Integrative Cancer Therapies. 2011 Sep;10(3):NP1-NP11.
- Smith JJ, Strombom P et al. Assessment of a watch-and-wait strategy for rectal cancer in patients with a complete response after neoadjuvant therapy. JAMA Oncology. 2019 Jan 10:e185896.
- Hanna TP, King WD et al. Mortality due to cancer treatment delay: systematic review and meta-analysis. BMJ. 2020 Nov 4;371:m4087; Reinberg S. Delaying cancer care costs lives. HealthDay. November 5, 2020. Viewed November 8, 2020.
- Inpatient Surgery Report 2019. The Leapfrog Group. Viewed September 4, 2019.
- Leeds IL, Meyers PM et al. Psychosocial risks are independently associated with cancer surgery outcomes in medically comorbid patients. Annals of Surgical Oncology. 2019 Apr;26(4):936-944.
- Harvie M. Nutritional supplements and cancer: potential benefits and proven harms. American Society of Clinical Oncology Educational Book. 2014;e478-e486.
- Harvie M. Nutritional supplements and cancer: potential benefits and proven harms. American Society of Clinical Oncology Educational Book. 2014;e478-e486.
- Harvie M. Nutritional supplements and cancer: potential benefits and proven harms. American Society of Clinical Oncology Educational Book. 2014;e478-e486.
- Papaioannou D, Cooper KL et al. Antioxidants in the chemoprevention of colorectal cancer and colorectal adenomas in the general population: a systematic review and meta-analysis. Colorectal Disease. 2011;13(10):1085–1099; Song M, Garrett WS, Chan AT. Nutrients, foods, and colorectal cancer prevention. Gastroenterology. 2015;148(6):1244-60.e16; Katona BW, Weiss JM. Chemoprevention of colorectal cancer. Gastroenterology. 2020;158(2):368-388.
- Bonelli L, Puntoni M et al. Antioxidant supplement and long-term reduction of recurrent adenomas of the large bowel. A double-blind randomized trial. Journal of Gastroenterology. 2013;48(6):698-705.
- Harvie M. Nutritional supplements and cancer: potential benefits and proven harms. American Society of Clinical Oncology Educational Book. 2014;e478-e486.
- Sakamoto J, Morita S et al. Efficacy of adjuvant immunochemotherapy with polysaccharide K for patients with curatively resected colorectal cancer: a meta-analysis of centrally randomized controlled clinical trials. Cancer Immunology, Immunotherapy: CII. 2006 Apr;55(4):404-11; Ramberg JE, Nelson ED, Sinnott RA. Immunomodulatory dietary polysaccharides: a systematic review of the literature. Nutrition Journal. 2010 Nov 18;9:54; Torisu M, Hayashi Y et al. Significant prolongation of disease-free period gained by oral polysaccharide K (PSK) administration after curative surgical operation of colorectal cancer. Cancer Immunology, Immunotherapy. 1990;31(5):261–268; Mitomi T, Tsuchiya S et al. Randomized, controlled study on adjuvant immunochemotherapy with PSK in curatively resected colorectal cancer. the Cooperative Study Group of Surgical Adjuvant Immunochemotherapy for Cancer of Colon and Rectum (Kanagawa). Diseases of the Colon and Rectum. 1992;35(2):123–130.
- Evidence-Based Monographs: Professional Resource: Coriolus Versicolor. Ottawa Integrative Cancer Centre. Viewed June 6, 2019.
- Ohwada S, Ikeya T et al. Adjuvant immunochemotherapy with oral Tegafur/Uracil plus PSK in patients with stage II or III colorectal cancer: a randomised controlled study. British Journal of Cancer. 2004 Mar 8;90(5):1003-10.
- Yamashita K, Ougolkov AV et al. Adjuvant immunochemotherapy with protein-bound polysaccharide K for colon cancer in relation to oncogenic beta-catenin activation. Diseases of the Colon and Rectum. 2007 Aug;50(8):1169-81.
- Sakai T, Yamashita Y, Maekawa T, Mikami K, Hoshino S, Shirakusa T. Immunochemotherapy with PSK and fluoropyrimidines improves long-term prognosis for curatively resected colorectal cancer. Cancer Biotherapy & Radiopharmaceuticals. 2008 Aug;23(4):461-7.
- Abrams DI, Weil AT. Integrative Oncology, 2nd Edition. New York, NY: Oxford University Press. 2014.
- Borchers AT, Stern JS, Hackman RM, Keen CL, Gershwin ME. Mushrooms, tumors, and immunity. Proceedings of the Society for Experimental Biology and Medicine. 1999;221(4):281–293.
- Li YH, Niu YB et al. Role of phytochemicals in colorectal cancer prevention. World Journal of Gastroenterology. 2015 Aug 21;21(31):9262-72.
- Alschuler LN, Gazella KA. The Definitive Guide to Cancer, 3rd Edition: An Integrative Approach to Prevention, Treatment, and Healing. Berkeley, California: Celestial Arts. 2010; Alschuler LN, Gazella KA. The Definitive Guide to Thriving after Cancer: A Five-Step Integrative Plan to Reduce the Risk of Recurrence and Build Lifelong Health. Berkeley, California: Ten Speed Press. 2013.
- McKinney N. Naturopathic Oncology, 3rd Edition. Victoria, BC, Canada: Liaison Press. 2016.
- Maalmi H, Ordóñez-Mena JM, Schöttker B, Brenner H. Serum 25-hydroxyvitamin D levels and survival in colorectal and breast cancer patients: systematic review and meta-analysis of prospective cohort studies. European Journal of Cancer. 2014 May;50(8):1510-21; Shang M, Sun J. Vitamin D/VDR, probiotics, and gastrointestinal diseases. Current Medicinal Chemistry. 2017;24(9):876-887.
- Urashima M, Ohdaira H et al. Effect of vitamin D supplementation on relapse-free survival among patients with digestive tract cancers: the AMATERASU randomized clinical trial. JAMA. 2019 Apr 9;321(14):1361-1369.
- Ng K, Nimeiri HS et al. Effect of high-dose vs standard-dose vitamin D3 supplementation on progression-free survival among patients with advanced or metastatic colorectal cancer: the SUNSHINE randomized clinical trial. JAMA. 2019 Apr 9;321(14):1370-1379.
- Lin S, An X, Guo Y, et al. Meta-analysis of astragalus-containing traditional Chinese medicine combined with chemotherapy for colorectal cancer: efficacy and safety to tumor response. Frontiers in Oncology. 2019;9:749; Abrams DI, Weil AT. Integrative Oncology, 2nd Edition. New York, NY: Oxford University Press. 2014; Chen M, May BH, Zhou IW, Xue CC, Zhang AL. FOLFOX 4 combined with herbal medicine for advanced colorectal cancer: a systematic review. Phytotherapy Research. 2014;28(7):976–991.
- Ong SKL, Shanmugam MK et al. Focus on formononetin: anticancer potential and molecular targets. Cancers (Basel). 2019;11(5):611; Dong J, Liang W et al. Saponins regulate intestinal inflammation in colon cancer and IBD. Pharmacological Research. 2019;144:66–72.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS Journal. 2013 Jan;15(1):195-218.
- Greil R, Greil-Ressler S et al. A phase 1 dose-escalation study on the safety, tolerability and activity of liposomal curcumin (Lipocurc™) in patients with locally advanced or metastatic cancer. Cancer Chemotherapy and Pharmacology. 2018 Oct;82(4):695-706.
- Sahebkar A. Are curcuminoids effective C-reactive protein-lowering agents in clinical practice? Evidence from a meta-analysis. Phytotherapy Research. 2014 May;28(5):633-42.
- Patel BB, Majumdar AP. Synergistic role of curcumin with current therapeutics in colorectal cancer: minireview. Nutrition and Cancer. 2009;61(6):842-6; Jalili-Nik M, Soltani A et al. Current status and future prospective of curcumin as a potential therapeutic agent in the treatment of colorectal cancer. Journal of Cellular Physiology. 2018 Sep;233(9):6337-6345.
- Howells LM, Iwuji COO et al. Curcumin combined with FOLFOX chemotherapy is safe and tolerable in patients with metastatic colorectal cancer in a randomized phase IIa trial. Journal of Nutrition. 2019 Jul 1;149(7):1133-1139.
- Ismail NI, Othman I, Abas F, H Lajis N, Naidu R.l. Mechanism of apoptosis induced by curcumin in colorectal cancer. International Journal of Molecular Sciiences. 2019 May 17;20(10). pii: E2454.
- Ravindran J, Prasad S, Aggarwal BB. Curcumin and cancer cells: How many ways can curry kill tumor cells selectively? AAPS J. 2009 Sep;11(3):495-510.
- Huang YF, Zhu DJ et al. Curcumin enhances the effects of irinotecan on colorectal cancer cells through the generation of reactive oxygen species and activation of the endoplasmic reticulum stress pathway. Oncotarget. 2017 Jun 20;8(25):40264-40275.
- Noratto GD, Jutooru I, Safe S, Angel-Morales G, Mertens-Talcott SU. The drug resistance suppression induced by curcuminoids in colon cancer SW-480 cells is mediated by reactive oxygen species-induced disruption of the microRNA-27a-ZBTB10-Sp axis. Molecular Nutrition and Food Research. 2013 Sep;57(9):1638-48.
- Bahrami A, Majeed M, Sahebkar A. Curcumin: a potent agent to reverse epithelial-to-mesenchymal transition. Cellular Oncology (Dordrecht). 2019 Aug;42(4):405-421.
- Alschuler LN, Gazella KA. The Definitive Guide to Cancer, 3rd Edition: An Integrative Approach to Prevention, Treatment, and Healing. Berkeley, California: Celestial Arts. 2010; Alschuler LN, Gazella KA. The Definitive Guide to Thriving after Cancer: A Five-Step Integrative Plan to Reduce the Risk of Recurrence and Build Lifelong Health. Berkeley, California: Ten Speed Press. 2013.
- Lemole G, Mehta P, McKee D. After Cancer Care: The Definitive Self-Care Guide to Getting and Staying Well for Patients with Cancer. New York, New York: Rodale, Inc. 2015.
- McKinney N. Naturopathic Oncology, 3rd Edition. Victoria, BC, Canada: Liaison Press. 2016.
- Abrams DI, Weil AT. Integrative Oncology, 2nd Edition. New York, NY: Oxford University Press. 2014.
- Mueller T, Voigt W. Fermented wheat germ extract—nutritional supplement or anticancer drug? Nutrition Journal. 2011 Sep 5;10:89; Yeend T, Robinson K, Lockwood C, McArthur A. The effectiveness of fermented wheat germ extract as an adjunct therapy in the treatment of cancer: a systematic review. JBI Library of Systematic Reviews. 2012;10(42 Suppl):1-12.
- Mueller T, Voigt W. Fermented wheat germ extract—nutritional supplement or anticancer drug? Nutrition Journal. 2011 Sep 5;10:89; Mueller T, Jordan K, Voigt W. Promising cytotoxic activity profile of fermented wheat germ extract (Avemar®) in human cancer cell lines. Journal of Experimental & Clinical Cancer Research. 2011 Apr 16;30(1):42.
- Mueller T, Voigt W. Fermented wheat germ extract—nutritional supplement or anticancer drug? Nutrition Journal. 2011 Sep 5;10:89; Yeend T, Robinson K, Lockwood C, McArthur A. The effectiveness of fermented wheat germ extract as an adjunct therapy in the treatment of cancer: a systematic review. JBI Library of Systematic Reviews. 2012;10(42 Suppl):1-12.
- Zhurakivska K, Troiano G et al. The effects of adjuvant fermented wheat germ extract on cancer cell lines: a systematic review. Nutrients. 2018 Oct 19;10(10):1546.
- Mueller T, Voigt W. Fermented wheat germ extract—nutritional supplement or anticancer drug? Nutrition Journal. 2011 Sep 5;10:89.
- Zhurakivska K, Troiano G et al. The effects of adjuvant fermented wheat germ extract on cancer cell lines: a systematic review. Nutrients. 2018 Oct 19;10(10):1546.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Parmar G, Kaczor T. Textbook of Naturopathic Oncology: A Desktop Guide of Integrative Cancer Care. 1st edition. Canada: Medicatrix Holdings Ltd. 2020.
- Liu C, Li P et al. Advances in the antagonism of epigallocatechin-3-gallate in the treatment of digestive tract tumors. Molecules. 2019 May 3;24(9). pii: E1726; Kumar N, Shibata D, Helm J, Coppola D, Malafa M. Green tea polyphenols in the prevention of colon cancer. Frontiers in Bioscience. 2007 Jan 1;12:2309-15; Liu C, Li P et al. Advances in the antagonism of epigallocatechin-3-gallate in the treatment of digestive tract tumors. Molecules. 2019 May 3;24(9). pii: E1726.
- Shimizu M, Adachi S, Masuda M, Kozawa O, Moriwaki H. Cancer chemoprevention with green tea catechins by targeting receptor tyrosine kinases. Molecular Nutrition & Food Research. 2011 Jun;55(6):832-43.
- Shimizu M, Fukutomi Y et al. Green tea extracts for the prevention of metachronous colorectal adenomas: a pilot study. Cancer Epidemiology, Biomarkers & Prevention. 2008 Nov;17(11):3020-5; Shin CM, Lee DH et al. Green tea extracts for the prevention of metachronous colorectal polyps among patients who underwent endoscopic removal of colorectal adenomas: a randomized clinical trial. Clinical Nutrition. 2018 Apr;37(2):452-458.
- Chen J, Huang XF. The signal pathways in azoxymethane-induced colon cancer and preventive implications. Cancer Biology & Therapy. 2009 Jul;8(14):1313-7.
- Fajardo AM, Piazza GA. Chemoprevention in gastrointestinal physiology and disease. Anti-inflammatory approaches for colorectal cancer chemoprevention. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2015 Jul 15;309(2):G59-70; Ullah MF, Bhat SH et al. Pharmacological intervention through dietary nutraceuticals in gastrointestinal neoplasia. Critical Reviews in Food Science and Nutrition. 2016 Jul 3;56(9):1501-18.
- Ullah MF, Bhat SH et al. Pharmacological intervention through dietary nutraceuticals in gastrointestinal neoplasia. Critical Reviews in Food Science and Nutrition. 2016 Jul 3;56(9):1501-18.
- Ullah MF, Bhat SH et al. Pharmacological intervention through dietary nutraceuticals in gastrointestinal neoplasia. Critical Reviews in Food Science and Nutrition. 2016 Jul 3;56(9):1501-18.
- Hu G, Zhang L, Rong Y, Ni X, Sun Y. Downstream carcinogenesis signaling pathways by green tea polyphenols: a translational perspective of chemoprevention and treatment for cancers. Current Drug Metabolism. 2014 Jan;15(1):14-22.
- Alschuler LN, Gazella KA. The Definitive Guide to Cancer, 3rd Edition: An Integrative Approach to Prevention, Treatment, and Healing. Berkeley, California: Celestial Arts. 2010; Alschuler LN, Gazella KA. The Definitive Guide to Thriving after Cancer: A Five-Step Integrative Plan to Reduce the Risk of Recurrence and Build Lifelong Health. Berkeley, California: Ten Speed Press. 2013.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Lemole G, Mehta P, McKee D. After Cancer Care: The Definitive Self-Care Guide to Getting and Staying Well for Patients with Cancer. New York, New York: Rodale, Inc. 2015.
- Lissoni P, Barni S et al. Decreased toxicity and increased efficacy of cancer chemotherapy using the pineal hormone melatonin in metastatic solid tumour patients with poor clinical status. European Journal of Cancer. 1999 Nov;35(12):1688-92.
- Barni S, Lissoni P et al. A randomized study of low-dose subcutaneous interleukin-2 plus melatonin versus supportive care alone in metastatic colorectal cancer patients progressing under 5-fluorouracil and folates. Oncology. 1995;52:243–245; Lissoni P, Brivio F et al. Neuroimmunomodulation in medical oncology: application of psychoneuroimmunology with subcutaneous low-dose IL-2 and the pineal hormone melatonin in patients with untreatable metastatic solid tumors. Anticancer Research. 2008 Mar-Apr;28(2B):1377-81.
- Cerea G, Vaghi M et al. Biomodulation of cancer chemotherapy for metastatic colorectal cancer: a randomized study of weekly low-dose irinotecan alone versus irinotecan plus the oncostatic pineal hormone melatonin in metastatic colorectal cancer patients progressing on 5-fluorouracil-containing combinations. Anticancer Research. 2003 Mar-Apr;23(2C):1951-4.
- Alschuler LN, Gazella KA. The Definitive Guide to Cancer, 3rd Edition: An Integrative Approach to Prevention, Treatment, and Healing. Berkeley, California: Celestial Arts. 2010; Alschuler LN, Gazella KA. The Definitive Guide to Thriving after Cancer: A Five-Step Integrative Plan to Reduce the Risk of Recurrence and Build Lifelong Health. Berkeley, California: Ten Speed Press. 2013.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Lemole G, Mehta P, McKee D. After Cancer Care: The Definitive Self-Care Guide to Getting and Staying Well for Patients with Cancer. New York, New York: Rodale, Inc. 2015.
- McKinney N. Naturopathic Oncology, 3rd Edition. Victoria, BC, Canada: Liaison Press. 2016.
- Parmar G, Kaczor T. Textbook of Naturopathic Oncology: A Desktop Guide of Integrative Cancer Care. 1st edition. Canada: Medicatrix Holdings Ltd. 2020.
- Friedel WE, Matthes H, Bock PR, Zänker KS. Systematic evaluation of the clinical effects of supportive mistletoe treatment within chemo- and/or radiotherapy protocols and long-term mistletoe application in nonmetastatic colorectal carcinoma: multicenter, controlled, observational cohort study. Journal of the Society for Integrative Oncology. 2009 Fall;7(4):137-45.
- Parmar G, Kaczor T. Textbook of Naturopathic Oncology: A Desktop Guide of Integrative Cancer Care. 1st edition. Canada: Medicatrix Holdings Ltd. 2020.
- Song M, Zhang X et al. Marine ω-3 polyunsaturated fatty acid intake and survival after colorectal cancer diagnosis. Gut. 2017 Oct;66(10):1790-1796.
- Adiamah A, Skořepa P, Weimann A, Lobo DN. The impact of preoperative immune modulating nutrition on outcomes in patients undergoing surgery for gastrointestinal cancer: a systematic review and meta-analysis. Annals of Surgery. 2019 Feb 26.
- de Aguiar Pastore Silva J, Emilia de Souza Fabre M, Waitzberg DL. Omega-3 supplements for patients in chemotherapy and/or radiotherapy: a systematic review. Clinical Nutrition. 2015 Jun;34(3):359-66.
- Cheng J, Ogawa K et al. Increased intake of n-3 polyunsaturated fatty acids elevates the level of apoptosis in the normal sigmoid colon of patients polypectomized for adenomas/tumors. Cancer Letters. 2003 Apr 10;193(1):17-24.
- Cockbain AJ, Volpato M et al. Anticolorectal cancer activity of the omega-3 polyunsaturated fatty acid eicosapentaenoic acid. Gut. 2014 Nov;63(11):1760-8.
- Cockbain AJ, Volpato M et al. Anticolorectal cancer activity of the omega-3 polyunsaturated fatty acid eicosapentaenoic acid. Gut. 2014 Nov;63(11):1760-8.
- Courtney ED, Matthews S et al. Eicosapentaenoic acid (EPA) reduces crypt cell proliferation and increases apoptosis in normal colonic mucosa in subjects with a history of colorectal adenomas. International Journal of Colorectal Disease. 2007 Jul;22(7):765-76.
- Alschuler LN, Gazella KA. The Definitive Guide to Cancer, 3rd Edition: An Integrative Approach to Prevention, Treatment, and Healing. Berkeley, California: Celestial Arts. 2010; Alschuler LN, Gazella KA. The Definitive Guide to Thriving after Cancer: A Five-Step Integrative Plan to Reduce the Risk of Recurrence and Build Lifelong Health. Berkeley, California: Ten Speed Press. 2013.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Lemole G, Mehta P, McKee D. After Cancer Care: The Definitive Self-Care Guide to Getting and Staying Well for Patients with Cancer. New York, New York: Rodale, Inc. 2015.
- McKinney N. Naturopathic Oncology, 3rd Edition. Victoria, BC, Canada: Liaison Press. 2016.
- Parmar G, Kaczor T. Textbook of Naturopathic Oncology: A Desktop Guide of Integrative Cancer Care. 1st edition. Canada: Medicatrix Holdings Ltd. 2020.
- Howells LM, Berry DP et al. Phase I randomized, double-blind pilot study of micronized resveratrol (SRT501) in patients with hepatic metastases--safety, pharmacokinetics, and pharmacodynamics. Cancer Prevention Research (Philadelphia, Pa.). 2011;4(9):1419-1425.
- Patel KR, Brown VA et al. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Research. 2010 Oct 1;70(19):7392-9.
- Schneider Y, Duranton B et al. Resveratrol inhibits intestinal tumorigenesis and modulates host-defense-related gene expression in an animal model of human familial adenomatous polyposis.Nutrition and Cancer. 2001;39(1):102-7; Cui X, Jin Y et al. Resveratrol suppresses colitis and colon cancer associated with colitis. Cancer Prevention Research (Philadelphia). 2010 Apr;3(4):549-59.
- Santandreu FM, Valle A, Oliver J, Roca P. Resveratrol potentiates the cytotoxic oxidative stress induced by chemotherapy in human colon cancer cells. Cell Physiology and Biochemistry. 2011;28(2):219-28.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Lemole G, Mehta P, McKee D. After Cancer Care: The Definitive Self-Care Guide to Getting and Staying Well for Patients with Cancer. New York, New York: Rodale, Inc. 2015.
- McKinney N. Naturopathic Oncology, 3rd Edition. Victoria, BC, Canada: Liaison Press. 2016.
- Tanaka S, Haruma K, Yoshihara M, Kajiyama G, Kira K, Amagase H, Chayama K. Aged garlic extract has potential suppressive effect on colorectal adenomas in humans. Journal of Nutrition. 2006 Mar;136(3 Suppl):821S-826S.
- Chen M, May BH, Zhou IW, Xue CC, Zhang AL. Meta-analysis of oxaliplatin-based chemotherapy combined with traditional medicines for colorectal cancer: contributions of specific plants to tumor response. Integrative Cancer Therapies. 2016;15(1):40–59.
- Huang S, Peng W et al. Kangai injection, a traditional Chinese medicine, improves efficacy and reduces toxicity of chemotherapy in advanced colorectal cancer patients: a systematic review and meta-analysis. Evidence-based Complementary and Alternative Medicine. 2019 Jul 15;2019:8423037.
- Yu R, Wu X, Jia L, Lou Y. Effect of Chinese herbal compound LC09 on patients with capecitabine-associated hand-foot syndrome: a randomized, double-blind, and parallel-controlled trial. Integrative Cancer Therapies. Jan-Dec 2020;19:1534735420928466.
- Chen WT, Yang TS et al. Effectiveness of a novel herbal agent MB-6 as a potential adjunct to 5-fluoracil-based chemotherapy in colorectal cancer. Nutrition Research. 2014 Jul;34(7):585-94.
- Chen M, May BH, Zhou IW, Xue CC, Zhang AL. Meta-analysis of oxaliplatin-based chemotherapy combined with traditional medicines for colorectal cancer: contributions of specific plants to tumor response. Integrative Cancer Therapies. 2016;15(1):40–59.
- Zhang T, Yang YF et al. Efficacy and safety of Quxie Capsule () in metastatic colorectal cancer: a double-blind randomized placebo controlled trial. Chinese Journal of Integrative Medicine. 2018;24(3):171–177; Zhang T, Yang YF et al. Efficacy of quxie capsule in metastatic colorectal cancer: update of a double-blind, randomized, placebo controlled trial. Journal of Clinical Oncology. 2019;37(15_supp); Yang YF, Chen ZX, Xu Y. [Randomized controlled study on effect of Quxie Capsule on the median survival time and quality of life in patients with advanced colorectal carcinoma] [Article in Chinese]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2008;28(2):111–114.
- Yang YF, Xu Y, Wu Y. Clinical randomized double-blinded controlled study on Quxie Capsule in reducing post-operational relapse and metastasis of colorectal cancer] [Article in Chinese]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2007;27(10):879–882.
- Chen D, Yang Y, Yang P. Quxie Capsule inhibits colon tumor growth partially through foxo1-mediated apoptosis and immune modulation. Integrative Cancer Therapies. 2019;18:1534735419846377.
- Yehia R, Saleh S, El Abhar H, Saad AS, Schaalan M. L-Carnosine protects against oxaliplatin-induced peripheral neuropathy in colorectal cancer patients: a perspective on targeting Nrf-2 and NF-κB pathways. Toxicology and Applied Pharmacology. 2019;365:41-50.
- Gupta N, Saleem A et al. Carbogen and nicotinamide increase blood flow and 5-fluorouracil delivery but not 5-fluorouracil retention in colorectal cancer metastases in patients. Clinical Cancer Research. 2006;12(10):3115–3123.
- El Halabi I, Bejjany R et al. Ascorbic acid in colon cancer: from the basic to the clinical applications. International Journal of Moleculal Sciences. 2018;19(9):2752.
- Murata A, Morishige F, Yamaguchi H. Prolongation of survival times of terminal cancer patients by administration of large doses of ascorbate. International Journal for Vitam and Nutrition Research. Supplement. 1982;23:103-13.
- El Halabi I, Bejjany R et al. Ascorbic acid in colon cancer: from the basic to the clinical applications. International Journal of Moleculal Sciences. 2018;19(9):2752
- Yang LC, Hsieh CC, Lu TJ, Lin WC. Structurally characterized arabinogalactan from Anoectochilus formosanus as an immuno-modulator against CT26 colon cancer in BALB/c mice. Phytomedicine. 2014 Apr 15;21(5):647-55.
- Hagmar B, Ryd W, Skomedal H. Arabinogalactan blockade of experimental metastases to liver by murine hepatoma. Invasion Metastasis. 1991;11(6):348-55.
- Derry MM, Raina K, Agarwal R, Agarwal C. Characterization of azoxymethane-induced colon tumor metastasis to lung in a mouse model relevant to human sporadic colorectal cancer and evaluation of grape seed extract efficacy. Experimental and Toxicologic Pathology. 2014 Aug;66(5-6):235-42.
- Kaur M, Singh RP, Gu M, Agarwal R, Agarwal C. Grape seed extract inhibits in vitro and in vivo growth of human colorectal carcinoma cells. Clinical Cancer Research. 2006 Oct 15;12(20 Pt 1):6194-202.
- Cheah KY, Howarth GS, Bastian SE. Grape seed extract dose-responsively decreases disease severity in a rat model of mucositis; concomitantly enhancing chemotherapeutic effectiveness in colon cancer cells. PLoS One. 2014 Jan 21;9(1):e85184.
- Kim DJ, Shin DH et al. Chemoprevention of colon cancer by Korean food plant components. Mutation Research. 2003 Feb-Mar;523-524:99-107.
- Maneikyte J, Bausys A et al. Dietary glycine decreases both tumor volume and vascularization in a combined colorectal liver metastasis and chemotherapy model. International Journal of Biological Sciences. 2019 Jun 4;15(8):1582-1590.
- Ranji P, Akbarzadeh A, Rahmati-Yamchi M. Associations of probiotics with vitamin D and leptin receptors and their effects on colon cancer. Asian Pacific Journal of Cancer Prevention. 2015;16(9):3621-7; Shang M, Sun J. Vitamin D/VDR, probiotics, and gastrointestinal diseases. Current Medicinal Chemistry. 2017;24(9):876-887.
- Albandar HJ, Markert R, Agrawal S. The relationship between aspirin use and mortality in colorectal cancer. Journal of Gastrointestinal Oncology. 2018 Dec;9(6):1133-1137; Bains SJ, Mahic M et al. Aspirin as secondary prevention in patients with colorectal cancer: an unselected population-based study. Journal of Clinical Oncology. 2016 Jul 20;34(21):2501-8.
- Li P, Wu H et al. Aspirin use after diagnosis but not prediagnosis improves established colorectal cancer survival: a meta-analysis. Gut. 2015 Sep;64(9):1419-25.
- Figueiredo JC, Jacobs EJ, Newton CC, Guinter MA, Cance WG, Campbell PT. Associations of aspirin and non-aspirin non-steroidal anti-inflammatory drugs with colorectal cancer mortality after diagnosis. NCI: Journal of the National Cancer Institute. 2021 Feb 2:djab008.
- Frouws MA, Reimers MS et al. The influence of BRAF and KRAS mutation status on the association between aspirin use and survival after colon cancer diagnosis.PLoS One. 2017 Jan 26;12(1):e0170775.
- Durko L, Malecka-Panas E et al. Lifestyle modifications and colorectal cancer. Current Colorectal Cancer Reports. 2014;10:45-54.
- Loomans-Kropp HA, Pinsky P, Cao Y, Chan AT, Umar A. Association of aspirin use with mortality risk among older adult participants in the prostate, lung, colorectal, and ovarian cancer screening trial. JAMA Network Open. 2019 Dec 2;2(12):e1916729.
- Mohammed A, Yarla NS, Madka V, Rao CV. Clinically relevant anti-inflammatory agents for chemoprevention of colorectal cancer: new perspectives. International Journal of Molecular Sciences. 2018 Aug 8;19(8). pii: E2332.
- Fong W, To KKW. Drug repurposing to overcome resistance to various therapies for colorectal cancer. Cellular and Molecular Life Sciences. 2019 Sep;76(17):3383-3406.
- Restivo A, Cocco IM et al. Aspirin as a neoadjuvant agent during preoperative chemoradiation for rectal cancer. British Journal of Cancer. 2015 Oct 20;113(8):1133-9; Elwood PC, Morgan G et al. Aspirin in the treatment of cancer: reductions in metastatic spread and in mortality: a systematic review and meta-analyses of published studies. PLoS One. 2016 Apr 20;11(4):e0152402.
- Fong W, To KKW. Drug repurposing to overcome resistance to various therapies for colorectal cancer. Cellular and Molecular Life Sciences. 2019;76(17):3383–3406.
- Fong W, To KKW. Drug repurposing to overcome resistance to various therapies for colorectal cancer. Cellular and Molecular Life Sciences. 2019 Sep;76(17):3383-3406.
- McNeil JJ, Gibbs P et al. Effect of aspirin on cancer incidence and mortality in older adults. Journal of the National Cancer Institute. 2020;djaa114.
- Wakeman C, Keenan J et al. Chemoprevention of colorectal neoplasia. ANZ Journal of Surgery. 2017 Dec;87(12):E228-E232.
- Gash KJ, Chambers AC, Cotton DE, Williams AC, Thomas MG. Potentiating the effects of radiotherapy in rectal cancer: the role of aspirin, statins and metformin as adjuncts to therapy. British Journal of Cancer. 2017 Jul 11;117(2):210-219.
- Restivo A, Cocco IM et al. Aspirin as a neoadjuvant agent during preoperative chemoradiation for rectal cancer. British Journal of Cancer. 2015 Oct 20;113(8):1133-9.
- Hardie C, Jung Y, Jameson M. Effect of statin and aspirin use on toxicity and pathological complete response rate of neo-adjuvant chemoradiation for rectal cancer. Asia-Pacific Journal of Clinical Oncology. 2016 Jun;12(2):167-73.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Chang R. Beyond the Magic Bullet: The Anti-Cancer Cocktail. New York: Square One Publishers. 2012.
- Lévi F, Zidani R, Misset JL. Randomised multicentre trial of chronotherapy with oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer. International Organization for Cancer Chronotherapy. Lancet. 1997 Sep 6;350(9079):681-6.
- Innominato PF, Roche VP et al. The circadian timing system in clinical oncology. Annals of Medicine. 2014 Jun;46(4):191-207; Giacchetti S, Dugué PA et al.Sex moderates circadian chemotherapy effects on survival of patients with metastatic colorectal cancer: a meta-analysis. Annals of Oncology. 2012 Dec;23(12):3110-6; Lévi F. Chronotherapeutics: the relevance of timing in cancer therapy. Cancer Causes & Control. 2006 May;17(4):611-21; Lévi F, Focan C et al. Implications of circadian clocks for the rhythmic delivery of cancer therapeutics. Advanced Drug Delivery Reviews. 2007;59(9-10):1015–1035; Liao C, Li J, Bin Q, Cao Y, Gao F. Chronomodulated chemotherapy versus conventional chemotherapy for advanced colorectal cancer: a meta-analysis of five randomized controlled trials. International Journal of Colorectal Disease. 2010 Mar;25(3):343-50; Mormont MC, Waterhouse J et al. Marked 24-h rest/activity rhythms are associated with better quality of life, better response, and longer survival in patients with metastatic colorectal cancer and good performance status. Clinical Cancer Research. 2000;6(8):3038–3045.
- Lis CG, Grutsch JF et al. Circadian timing in cancer treatment: the biological foundation for an integrative approach. Integrative Cancer Therapies. 2003 Jun;2(2):105-11.
- Lévi F, Zidani R, Misset JL. Randomised multicentre trial of chronotherapy with oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer. International Organization for Cancer Chronotherapy. Lancet. 1997;350(9079):681–686.
- Lévi FA, Zidani R et al. Chronomodulated versus fixed-infusion-rate delivery of ambulatory chemotherapy with oxaliplatin, fluorouracil, and folinic acid (leucovorin) in patients with colorectal cancer metastases: a randomized multi-institutional trial. Journal of the National Cancer Institute. 1994 Nov 2;86(21):1608-17.
- Giacchetti S, Bjarnason G et al. First line infusion of 5-fluorouracil, leucovorin and oxaliplatin for metastatic colorectal cancer: 4-day chronomodulated (FFL4–10) versus 2-day FOLFOX2. A multicenter randomized phase III trial of the Chronotherapy Group of the European Organization for Research and Treatment of Cancer (EORTC 05963). Journal of Clinical Oncology. 2004 Jul 15;22(14_suppl):3526-3526.
- Lévi F, Focan C et al. Implications of circadian clocks for the rhythmic delivery of cancer therapeutics. Advanced Drug Delivery Reviews. 2007;59(9-10):1015–1035.
- Lévi F, Karaboué A et al. Cetuximab and circadian chronomodulated chemotherapy as salvage treatment for metastatic colorectal cancer (mCRC): safety, efficacy and improved secondary surgical resectability. Cancer Chemotherapy and Pharmacology. 2011;67(2):339–348; Garufi C, Torsello A et al. Cetuximab plus chronomodulated irinotecan, 5-fluorouracil, leucovorin and oxaliplatin as neoadjuvant chemotherapy in colorectal liver metastases: POCHER trial. British Journal of Cancer. 2010 Nov 9;103(10):1542-7; Garufi C, Torsello A et al. Cetuximab plus chronomodulated irinotecan, 5-fluorouracil, leucovorin and oxaliplatin as neoadjuvant chemotherapy in colorectal liver metastases: POCHER trial. British Journal of Cancer. 2010 Nov 9;103(10):1542-7.
- Block KI, Block PB et al. Making circadian cancer therapy practical. Integrative Cancer Therapies. 2009 Dec;8(4):371-86.
- Lévi F, Focan C et al.Implications of circadian clocks for the rhythmic delivery of cancer therapeutics. Advanced Drug Delivery Reviews. 2007;59(9-10):1015–1035.
- Jacobs BA, Deenen MJ et al. Pronounced between-subject and circadian variability in thymidylate synthase and dihydropyrimidine dehydrogenase enzyme activity in human volunteers. British Journal of Clinical Pharmacology. 2016 Sep;82(3):706-16.
- Baker D. Application of chronotherapy to the treatment of cancer: can changing the timing of drug administration influence efficacy and toxicity? Advances in Pharmacy. 2004 Jul;2(3):222-228.
- Lee JH, Kim TI et al. The effects of metformin on the survival of colorectal cancer patients with diabetes mellitus. International Journal of Cancer. 2012;131:752–759; Yang IP, Miao ZF et al. High blood sugar levels but not diabetes mellitus significantly enhance oxaliplatin chemoresistance in patients with stage III colorectal cancer receiving adjuvant FOLFOX6 chemotherapy. Therapeutic Advanced in Medical Oncology. 2019 Aug 20;11:1758835919866964.
- Mafiana RN, Al-Kindi MS, Mafiana N, Al Lawati AS, Al Moundhri M. Impact of Metabolic syndrome diagnosis and its treatment on survival of colorectal cancer patients. Journal of Cancer Epidemiology. 2019 Apr 21;2019:6527457.
- Gash KJ, Chambers AC, Cotton DE, Williams AC, Thomas MG. Potentiating the effects of radiotherapy in rectal cancer: the role of aspirin, statins and metformin as adjuncts to therapy. British Journal of Cancer. 2017 Jul 11;117(2):210-219.
- Bragagnoli A, Araujo R et al. Final results of a phase II of metformin plus irinotecan for refractory colorectal cancer. Journal of Clinical Oncology. 2018;36(15_suppl):e15527-e15527.
- Miranda VC, Braghiroli MI et al. Phase 2 trial of metformin combined with 5-fluorouracil in patients with refractory metastatic colorectal cancer. Clinical Colorectal Cancer. 2016 Dec;15(4):321-328.e1.
- Yang IP, Miao ZF et al. High blood sugar levels but not diabetes mellitus significantly enhance oxaliplatin chemoresistance in patients with stage III colorectal cancer receiving adjuvant FOLFOX6 chemotherapy. Therapeutic Advanced in Medical Oncology. 2019 Aug 20;11:1758835919866964.
- Coyle C, Cafferty FH, Vale C, Langley RE. Metformin as an adjuvant treatment for cancer: a systematic review and meta-analysis. Annals of Oncology. 2016;27(12):2184-2195.
- Eikawa S, Nishida M et al. Immune-mediated antitumor effect by type 2 diabetes drug, metformin. Proceedings of the National Academy of Sciences of the United States of America. 2015 Feb 10;112(6):1809-14.
- Cha JH, Yang WH et al. Metformin promotes antitumor immunity via endoplasmic-reticulum-associated degradation of PD-L1. Molecular Cell. 2018 Aug 16;71(4):606-620.e7.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Mei Z, Liang M et al. Effects of statins on cancer mortality and progression: a systematic review and meta-analysis of 95 cohorts including 1,111,407 individuals. International Journal of Cancer. 2017 Mar 1;140(5):1068-1081; He Y, Li X et al. Statins and multiple noncardiovascular outcomes: umbrella review of meta-analyses of observational studies and randomized controlled trials. Annals of Internal Medicine. 2018 Oct 16;169(8):543-553.
- Yokomichi H, Nagai A et al. Statin use and all-cause and cancer mortality: BioBank Japan cohort. Journal of Epidemiology. 2017 Mar;27(3S):S84-S91.
- Li Y, He X, Ding Y, Chen H, Sun L. Statin uses and mortality in colorectal cancer patients: An updated systematic review and meta-analysis. Cancer Medicine. 2019;8(6):3305–3313; Zhong S, Zhang X et al. Statin use and mortality in cancer patients: systematic review and meta-analysis of observational studies. Cancer Treatment Reviews. 2015 Jun;41(6):554-67; Ling Y, Yang L et al. Prognostic significance of statin use in colorectal cancer: a systematic review and meta-analysis. Medicine (Baltimore). 2015 Jun;94(25):e908; Cai H, Zhang G, Wang Z, Luo Z, Zhou X. Relationship between the use of statins and patient survival in colorectal cancer: a systematic review and meta-analysis. PLoS One. 2015 Jun 1;10(6):e0126944; Gray RT, Coleman HG, Hughes C, Murray LJ, Cardwell CR. Statin use and survival in colorectal cancer: results from a population-based cohort study and an updated systematic review and meta-analysis. Cancer Epidemiology. 2016 Dec;45:71-81.
- Mafiana RN, Al-Kindi MS, Mafiana N, Al Lawati AS, Al Moundhri M. Impact of metabolic syndrome diagnosis and its treatment on survival of colorectal cancer patients. Journal of Cancer Epidemiology. 2019;2019:6527457.
- Joo MK, Park JJ, Chun HJ. Additional benefits of routine drugs on gastrointestinal cancer: statins, metformin, and proton pump inhibitors. Digestive Diseases. 2018;36(1):1-14.
- Qu B, Qu H. The influence of statins on risk and patient survival in colorectal cancer. Journal of Clinical Gastroenterology. 2019;53(9):699–701.
- Fransgaard T, Hallas J, Thygesen LC, Gögenur I. Association of statin use and oncological outcomes after neoadjuvant radiotherapy in patients with rectal cancer. Anticancer Research. 2019;39(4):2177–2182.
- Rutledge BP, Desai P et al. The association between statins and colorectal cancer stage in the Women's Health Initiative. Molecular and Clinical Oncology. 2019;11(3):252–258.
- Coogan PF, Smith J, Rosenberg L. Statin use and risk of colorectal cancer. Journal of the National Cancer Institute. 2007;99(1):32–40.
- Gash KJ, Chambers AC, Cotton DE, Williams AC, Thomas MG. Potentiating the effects of radiotherapy in rectal cancer: the role of aspirin, statins and metformin as adjuncts to therapy. British Journal of Cancer. 2017 Jul 11;117(2):210-219.
- Qu B, Qu H. The influence of statins on risk and patient survival in colorectal cancer. Journal of Clinical Gastroenterology. 2019;53(9):699–701.
- Qu B, Qu H. The influence of statins on risk and patient survival in colorectal cancer. Journal of Clinical Gastroenterology. 2019;53(9):699–701; Krens LL, Simkens LH, Baas JM, et al. Statin use is not associated with improved progression free survival in cetuximab treated KRAS mutant metastatic colorectal cancer patients: results from the CAIRO2 study. PLoS One. 2014;9(11):e112201.
- Fong W, To KKW. Drug repurposing to overcome resistance to various therapies for colorectal cancer. Cellular and Molecular Life Sciences. 2019;76(17):3383–3406.
- Fong W, To KKW. Drug repurposing to overcome resistance to various therapies for colorectal cancer. Cellular and Molecular Life Sciences. 2019;76(17):3383–3406.
- Ahn KS, Sethi G, Aggarwal BB. Reversal of chemoresistance and enhancement of apoptosis by statins through down-regulation of the NF-kappaB pathway. Biochemical pharmacology. 2008 Feb 15;75(4):907-13.
- Agarwal B, Bhendwal S et al. Lovastatin augments apoptosis induced by chemotherapeutic agents in colon cancer cells. Clinical Cancer Research. 1999;5(8):2223–2229.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Krishna S, Ganapathi S et al. A randomised, double blind, placebo-controlled pilot study of oral artesunate therapy for colorectal cancer. EBioMedicine. 2014 Nov 15;2(1):82-90.
- Raffetin A, Bruneel F et al. Use of artesunate in non-malarial indications. Médecine et Maladies Infectieuses. 2018;48(4):238–249.
- Wang LL, Kong L, Liu H, et al. Design and synthesis of novel artemisinin derivatives with potent activities against colorectal cancer in vitro and in vivo. European Journal of Medicinal Chemistry. 2019;182:111665.
- Yao Z, Bhandari A, Wang Y, et al. Dihydroartemisinin potentiates antitumor activity of 5-fluorouracil against a resistant colorectal cancer cell line. Biochemical and Biophysical Research Communications. 2018;501(3):636–642.
- Kang XJ, Wang HY, Peng HG, et al. Codelivery of dihydroartemisinin and doxorubicin in mannosylated liposomes for drug-resistant colon cancer therapy. Acta Pharmacol Sin. 2017;38(6):885–896.
- Lee DH, Hasanuzzaman M et al. 10-phenylpyrazole artemisinin is a novel P-glycoprotein inhibitor that suppresses the overexpression and function of P-glycoprotein. Current Pharmaceutical Design. 2018;24(46):5590–5597.
- Parmar G, Kaczor T. Textbook of Naturopathic Oncology: A Desktop Guide of Integrative Cancer Care. 1st edition. Canada: Medicatrix Holdings Ltd. 2020.
- Losurdo G, Principi M et al. Histamine and histaminergic receptors in colorectal cancer: from basic science to evidence-based medicine. Anti-Cancer Agents in Medicinal Chemistry. 2018;18(1):15–20.
- Deva S, Jameson M. Histamine type 2 receptor antagonists as adjuvant treatment for resected colorectal cancer. Cochrane Database of Systematic Reviews. 2012;(8):CD007814; Pantziarka P, Bouche G, Meheus L, Sukhatme V, Sukhatme VP. Repurposing drugs in oncology (ReDO)—cimetidine as an anti-cancer agent. Ecancermedicalscience. 2014;8:485; Kelly MD, King J et al. Randomized trial of preoperative cimetidine in patients with colorectal carcinoma with quantitative assessment of tumor-associated lymphocytes. Cancer. 1999 Apr 15;85(8):1658-63.
- Svendsen LB, Ross C et al. Cimetidine as an adjuvant treatment in colorectal cancer. A double-blind, randomized pilot study. Diseases of the Colon and Rectum. 1995 May;38(5):514-8; Jameson MB, Michael Arendse, Pillai A et al. Final analysis of a randomized placebo-controlled double-blind phase II trial of perioperative cimetidine (CIM) in early colorectal cancer (CRC). Journal of Clinical Oncology. 2018;36(15_suppl):e15678-e15678; Yoshimatsu K, Ishibashi K et al. [Can the survival of patients with recurrent disease after curative resection of colorectal cancer be prolonged by the administration of cimetidine?] [Article in Japanese]. Gan To Kagaku Ryoho. 2006 Nov;33(12):1730-2.
- Pantziarka P, Bouche G, Meheus L, Sukhatme V, Sukhatme VP. Repurposing drugs in oncology (ReDO)—cimetidine as an anti-cancer agent. Ecancermedicalscience. 2014;8:485.
- Lin CY, Bai DJ, Yuan HY, et al. Perioperative cimetidine administration promotes peripheral blood lymphocytes and tumor infiltrating lymphocytes in patients with gastrointestinal cancer: results of a randomized controlled clinical trial. World Journal of Gastroenterology. 2004;10(1):136–142.
- Losurdo G, Principi M et al. Histamine and histaminergic receptors in colorectal cancer: from basic science to evidence-based medicine. Anti-Cancer Agents in Medicinal Chemistry. 2018;18(1):15–20; Pantziarka P, Bouche G, Meheus L, Sukhatme V, Sukhatme VP. Repurposing drugs in oncology (ReDO)—cimetidine as an anti-cancer agent. Ecancermedicalscience. 2014;8:485.
- Losurdo G, Principi M et al. Histamine and histaminergic receptors in colorectal cancer: from basic science to evidence-based medicine. Anti-Cancer Agents in Medicinal Chemistry. 2018;18(1):15–20; Pantziarka P, Bouche G, Meheus L, Sukhatme V, Sukhatme VP. Repurposing drugs in oncology (ReDO)—cimetidine as an anti-cancer agent. Ecancermedicalscience. 2014;8:485.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Chang R. Beyond the Magic Bullet: The Anti-Cancer Cocktail. New York: Square One Publishers. 2012.
- Fong W, To KKW. Drug repurposing to overcome resistance to various therapies for colorectal cancer. Cellular and Molecular Life Sciences. 2019;76(17):3383–3406; Mahalingam D, Mita M et al. Combined autophagy and HDAC inhibition: a phase I safety, tolerability, pharmacokinetic, and pharmacodynamic analysis of hydroxychloroquine in combination with the HDAC inhibitor vorinostat in patients with advanced solid tumors. Autophagy. 2014;10(8):1403–1414.
- Sasaki K, Tsuno NH et al. Chloroquine potentiates the anti-cancer effect of 5-fluorouracil on colon cancer cells. BMC Cancer. 2010;10:370.
- Fong W, To KKW. Drug repurposing to overcome resistance to various therapies for colorectal cancer. Cellular and Molecular Life Sciences. 2019;76(17):3383–3406; Marinković M, Šprung M, Buljubašić M, Novak I. Autophagy modulation in cancer: current knowledge on action and therapy. Oxidative Medicine and Cellular Longevity. 2018;2018:8023821; Schonewolf CA, Mehta M, Schiff D, et al. Autophagy inhibition by chloroquine sensitizes HT-29 colorectal cancer cells to concurrent chemoradiation. World Journal of Gastrointestinal Oncology. 2014;6(3):74–82.
- Gartner EM, Griffith KA et al. A pilot trial of the anti-angiogenic copper lowering agent tetrathiomolybdate in combination with irinotecan, 5-flurouracil, and leucovorin for metastatic colorectal cancer. Investigational New Drugs. 2009;27(2):159–165.
- Khan G, Merajver S. Copper chelation in cancer therapy using tetrathiomolybdate: an evolving paradigm. Expert Opinion on Investigational Drugs. 2009;18(4):541–548; Brewer GJ. Anticopper therapy against cancer and diseases of inflammation and fibrosis. Drug Discovery Today. 2005;10(16):1103–1109.
- Fatfat M, Merhi RA et al. Copper chelation selectively kills colon cancer cells through redox cycling and generation of reactive oxygen species. BMC Cancer. 2014;14:527.
- Baldari S, Di Rocco G et al. Effects of copper chelation on BRAFV600E positive colon carcinoma cells. Cancers (Basel). 2019;11(5):659.
- Rolim PM, Fidelis GP et al. Phenolic profile and antioxidant activity from peels and seeds of melon (Cucumis melo L. var. reticulatus) and their antiproliferative effect in cancer cells. Brazilian Journal of Medical and Biological Research. 2018;51(4):e6069.
- Yu N, Zhu H et al. Combination of Fe/Cu -chelators and docosahexaenoic acid: an exploration for the treatment of colorectal cancer. Oncotarget. 2017;8(31):51478–51491.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Parmar G, Kaczor T. Textbook of Naturopathic Oncology: A Desktop Guide of Integrative Cancer Care. 1st edition. Canada: Medicatrix Holdings Ltd. 2020.
- Meyn RE, Krishnan S, Skinner HD. Everything old is new again: using nelfinavir to radiosensitize rectal cancer. Clinical Cancer Research. 2016;22(8):1834–1836; Hill EJ, Roberts C et al. Clinical trial of oral nelfinavir before and during radiation therapy for advanced rectal cancer. Clinical Cancer Research. 2016;22(8):1922–1931.
- Buijsen J, Lammering G, Jansen RL, et al. Phase I trial of the combination of the Akt inhibitor nelfinavir and chemoradiation for locally advanced rectal cancer. Radiotherapy & Oncology. 2013;107(2):184–188.
- Bernstein WB, Dennis PA. Repositioning HIV protease inhibitors as cancer therapeutics. Current Opinion in HIV and AIDS. 2008;3(6):666–675
- Gills JJ, Lopiccolo J, Dennis PA. Nelfinavir, a new anti-cancer drug with pleiotropic effects and many paths to autophagy. Autophagy. 2008;4(1):107–109.
- Meyerhardt JA, Shi Q. Effect of celecoxib vs placebo added to standard adjuvant therapy on disease-free survival among patients with stage III colon cancer: the CALGB/SWOG 80702 (Alliance) randomized clinical trial. JAMA. 2021 Apr 6;325(13):1277-1286.
- Fong W, To KKW. Drug repurposing to overcome resistance to various therapies for colorectal cancer. Cellular and Molecular Life Sciences. 2019;76(17):3383–3406.
- Fong W, To KKW. Drug repurposing to overcome resistance to various therapies for colorectal cancer. Cellular and Molecular Life Sciences. 2019;76(17):3383–3406.
- Fong W, To KKW. Drug repurposing to overcome resistance to various therapies for colorectal cancer. Cellular and Molecular Life Sciences. 2019;76(17):3383–3406
- Fong W, To KKW. Drug repurposing to overcome resistance to various therapies for colorectal cancer. Cellular and Molecular Life Sciences. 2019;76(17):3383–3406.
- Pantziarka P, Sukhatme V, Bouche G, Meheus L, Sukhatme VP. Repurposing Drugs in Oncology (ReDO)-diclofenac as an anti-cancer agent. Ecancermedicalscience. 2016;10:610.
- Chi KH, Ko HL et al. Addition of rapamycin and hydroxychloroquine to metronomic chemotherapy as a second line treatment results in high salvage rates for refractory metastatic solid tumors: a pilot safety and effectiveness analysis in a small patient cohort. Oncotarget. 2015;6(18):16735–16745.
- Cohen EE, Sharma MR et al. A phase I study of sirolimus and bevacizumab in patients with advanced malignancies. European Journal of Cancer. 2011;47(10):1484–1489.
- Buck E, Eyzaguirre A et al. Rapamycin synergizes with the epidermal growth factor receptor inhibitor erlotinib in non-small-cell lung, pancreatic, colon, and breast tumors. Molecular Cancer Therapeutics. 2006;5(11):2676–2684.
- Bader JE, Enos RT et al. Macrophage depletion using clodronate liposomes decreases tumorigenesis and alters gut microbiota in the AOM/DSS mouse model of colon cancer. American Journal of Physiology—Gastrointestinal and Liver Physiology. 2018;314(1):G22–G31.
- Baranyi M, Rittler D et al. Next generation lipophilic bisphosphonate shows antitumor effect in colorectal cancer in vitro and in vivo. Pathology Oncology Research. 2020;10.1007/s12253-019-00789-9.
- Zhu J, Liu M et al. Zoledronic acid regulates autophagy and induces apoptosis in colon cancer cell line CT26. BioMed Research International. 2017;2017:7203584; Peng Y, Qiu L et al. M4IDP, a zoledronic acid derivative, induces G1 arrest, apoptosis and autophagy in HCT116 colon carcinoma cells via blocking PI3K/Akt/mTOR pathway. Life Sciences. 2017;185:63–72.
- Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Research Reviews. 2017;39:46–58.
- Lévesque S, Pol JG et al. Trial watch: dietary interventions for cancer therapy. Oncoimmunology. 2019;8(7):1591878.
- Di Tano M, Raucci F et al. Synergistic effect of fasting-mimicking diet and vitamin C against KRAS mutated cancers. Nature Communications. 2020;11(1):2332.
- Scheubeck G, Berchtold S et al. Starvation-induced differential virotherapy using an oncolytic measles vaccine virus. Viruses. 2019;11(7):614.
- Sun P, Wang H, He Z, et al. Fasting inhibits colorectal cancer growth by reducing M2 polarization of tumor-associated macrophages. Oncotarget. 2017;8(43):74649–74660.
- Lee BR, Kim HR et al. Enhanced therapeutic treatment of colorectal cancer using surface-modified nanoporous acupuncture needles. Scientific Reports. 2017;7(1):12900.
- Hager ED, Dziambor H et al. Deep hyperthermia with radiofrequencies in patients with liver metastases from colorectal cancer. Anticancer Research. 1999;19(4C):3403-3408.
- Davenport L. CRC with peritoneal metastases: CRS-HIPEC refinements. Medscape Oncology. July 6, 2020. Viewed July 13, 2020.
- Berdov BA, Menteshashvili GZ. Thermoradiotherapy of patients with locally advanced carcinoma of the rectum. International Journal of Hyperthermia. 1990;6(5):881-890.
- Quénet F, Elias D et al; UNICANCER-GI Group and BIG Renape Group. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021 Jan 18:S1470-2045(20)30599-4.
- Hegewisch-Becker S, Gruber Y et al. Whole-body hyperthermia (41.8 C) combined with bimonthly oxaliplatin, high-dose leucovorin and 5-fluorouracil 48-hour continuous infusion in pretreated metastatic colorectal cancer: a phase II study. Annals of Oncology. 2002;13(8):1197–1204.;Hildebrandt B, Drager J et al. Whole-body hyperthermia in the scope of von Ardenne’s systemic cancer multistep therapy (sCMT) combined with chemotherapy in patients with metastatic colorectal cancer: a phase I/II study. International Journal of Hyperthermia. 2004;20(3):317–333.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Care. New York: Bantam Dell. 2009. p. 329.
- Dhanapal R, Saraswathi T, Govind RN. Cancer cachexia. Journal of Oral and Maxillofacial Pathology. 2011 Sep;15(3):257-60.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Care. New York: Bantam Dell. 2009. p. 329.
- Denlinger CS, Barsevick AM et al. The challenges of colorectal cancer survivorship. Journal of the National Comprehensive Cancer Network. 2009 Sep;7(8):883-93; quiz 894.
- Pulsed low-dose pelvic reirradiation safe, provided tangible benefit for patients with few other options. Fox Chase Cancer Center, September 22, 2020. Viewed September 30, 2020; Paly JJ, Deng M et al. Pelvic reirradiation utilizing pulsed low-dose rate radiation therapy. American Journal of Clinical Oncology. 2020 Oct;43(10):748-751.
- Lin S, An X et al. Meta-analysis of astragalus-containing traditional Chinese medicine combined with chemotherapy for colorectal cancer: efficacy and safety to tumor response. Frontiers in Oncology. 2019;9:749; Taixiang W, Munro AJ, Guanjian L. Chinese medical herbs for chemotherapy side effects in colorectal cancer patients. Cochrane Database of Systematic Reviews. 2005;(1):CD004540; Chen M, May BH, Zhou IW, Xue CC, Zhang AL. FOLFOX 4 combined with herbal medicine for advanced colorectal cancer: a systematic review. Phytotherapy Research. 2014;28(7):976–991.
- Chen M, May BH et al. Oxaliplatin-based chemotherapy combined with traditional medicines for neutropenia in colorectal cancer: a meta-analysis of the contributions of specific plants. Critical Reviews in Oncology/Hematology. 2016;105:18–34.
- Huang WC, Kuo KT, Bamodu OA, et al. Astragalus polysaccharide (PG2) ameliorates cancer symptom clusters, as well as improves quality of life in patients with metastatic disease, through modulation of the inflammatory cascade. Cancers (Basel). 2019;11(8).
- Chen MH, May BH, Zhou IW, Zhang AL, Xue CC. Integrative medicine for relief of nausea and vomiting in the treatment of colorectal cancer using oxaliplatin-based chemotherapy: a systematic review and meta-analysis. Phytotherapy Research. 2016;30(5):741–753.
- Abrams DI, Weil AT. Integrative Oncology, 2nd Edition. New York, NY: Oxford University Press. 2014.
- Lin S, An X et al. Meta-analysis of astragalus-containing traditional Chinese medicine combined with chemotherapy for colorectal cancer: efficacy and safety to tumor response. Frontiers in Oncology. 2019;9:749; Taixiang W, Munro AJ, Guanjian L. Chinese medical herbs for chemotherapy side effects in colorectal cancer patients. Cochrane Database of Systematic Reviews. 2005;(1):CD004540.
- Dong J, Liang W et al. Saponins regulate intestinal inflammation in colon cancer and IBD. Pharmacological Research. 2019;144:66–72.
- Chen D, Zhao J, Cong W. Chinese herbal medicines facilitate the control of chemotherapy-induced side effects in colorectal cancer: progress and perspective. Frontiers in Pharmacology. 2018;9:1442.
- Hao J, Zhu X, Bensoussan A. Effects of nonpharmacological interventions in chemotherapy-induced peripheral neuropathy: an overview of systematic reviews and meta-analyses. Integrative Cancer Therapies. Jan-Dec 2020;19:1534735420945027; Noh H, Yoon SW, Park B. A systematic review of herbal medicine for chemotherapy-induced peripheral neuropathy (CIPN). Evidence Based Complementary and Alternative Medicine. 2018 Feb 14;2018:6194184; Wei X, Zhu L, Wang H, Wang C, Deng Q, Li X. Efficacy of traditional Chinese medicines in preventing oxaliplatin-induced peripheral neurotoxicity in cancer patients: a network meta-analysis. Chinese Herb Med. 2017;9(2):161-168; Deng B, Jia L, Cheng Z. Radix astragali-based Chinese herbal medicine for oxaliplatin-induced peripheral neuropathy: a systematic review and meta-analysis. Evidence-based Complementary and Alternative Medicine. 2016;2016:2421876.
- Lin S, An X et al. Meta-analysis of astragalus-containing traditional Chinese medicine combined with chemotherapy for colorectal cancer: efficacy and safety to tumor response. Frontiers in Oncology. 2019;9:749; Taixiang W, Munro AJ, Guanjian L. Chinese medical herbs for chemotherapy side effects in colorectal cancer patients. Cochrane Database of Systematic Reviews. 2005;(1):CD004540; Huang S, Peng W et al. Kangai injection, a traditional Chinese medicine, improves efficacy and reduces toxicity of chemotherapy in advanced colorectal cancer patients: a systematic review and meta-analysis. Evidence-based Complementary and Alternative Medicine. 2019 Jul 15;2019:8423037.
- Panahia Y, Saadat A et al. Antioxidant effects of bioavailability-enhanced curcuminoids in patients with solid tumors: a randomized double-blind placebo-controlled trial. Journal of Functional Foods. 2014 Jan;6:615-622; Panahi Y, Saadat A, Beiraghdar F, Sahebkar A. Adjuvant therapy with bioavailability-boosted curcuminoids suppresses systemic inflammation and improves quality of life in patients with solid tumors: a randomized double-blind placebo-controlled trial. Phytotherapy Research. 2014 Oct;28(10):1461-7.
- Yeung KS, Gubili J, Mao JJ. Herb-drug interactions in cancer care. Oncology (Williston Park). 2018 Oct 15;32(10):516-20; Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS Journal . 2013 Jan;15(1):195-218.
- He ZY, Shi CB et al. Upregulation of p53 expression in patients with colorectal cancer by administration of curcumin. Cancer Invest. 2011 Mar;29(3):208-13; Lagoa R, Silva J, Rodrigues JR, Bishayee A. Advances in phytochemical delivery systems for improved anticancer activity. Biotechnology Advances. 2019 Apr 9. pii: S0734-9750(19)30063-1; Amalraj A, Pius A, Gopi S, Gopi S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—a review. Journal of Traditional and Complementary Medicine. 2016 Jun 15;7(2):205-233; Belcaro G, Hosoi M et al. A controlled study of a lecithinized delivery system of curcumin (Meriva®) to alleviate the adverse effects of cancer treatment. Phytotherapy Research. 2014 Mar;28(3):444-50; Mansouri K, Rasoulpoor S et al. Clinical effects of curcumin in enhancing cancer therapy: a systematic review. BMC Cancer. 2020;20(1):791.
- Lagoa R, Silva J, Rodrigues JR, Bishayee A. Advances in phytochemical delivery systems for improved anticancer activity. Biotechnology Advances. 2019 Apr 9. pii: S0734-9750(19)30063-1; Amalraj A, Pius A, Gopi S, Gopi S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—a review. Journal of Traditional and Complementary Medicine. 2016 Jun 15;7(2):205-233; Belcaro G, Hosoi M et al. A controlled study of a lecithinized delivery system of curcumin (Meriva®) to alleviate the adverse effects of cancer treatment. Phytotherapy Research. 2014 Mar;28(3):444-50; Mansouri K, Rasoulpoor S et al. Clinical effects of curcumin in enhancing cancer therapy: a systematic review. BMC Cancer. 2020;20(1):791; Normando AGC, Menêses AG et al. Effects of turmeric and curcumin on oral mucositis: A systematic review. Phytherapy Research. 2019;33(5):1318-1329; Delavarian Z, Pakfetrat A et al. Oral administration of nanomicelle curcumin in the prevention of radiotherapy-induced mucositis in head and neck cancers. Special Care in Dentistry. 2019;39(2):166-172; Akbari S, Kariznavi E, Jannati M, Elyasi S, Tayarani-Najaran Z. Curcumin as a preventive or therapeutic measure for chemotherapy and radiotherapy induced adverse reaction: a comprehensive review. Food and Chemical Toxicology. 2020 Nov;145:111699.
- Belcaro G, Hosoi M et al. A controlled study of a lecithinized delivery system of curcumin (Meriva®) to alleviate the adverse effects of cancer treatment. Phytotherapy Research. 2014 Mar; 28:444-50.
- Lagoa R, Silva J, Rodrigues JR, Bishayee A. Advances in phytochemical delivery systems for improved anticancer activity. Biotechnology Advances. 2019 Apr 9. pii: S0734-9750(19)30063-1; Amalraj A, Pius A, Gopi S, Gopi S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—a review. Journal of Traditional and Complementary Medicine. 2016 Jun 15;7(2):205-233; Belcaro G, Hosoi M et al. A controlled study of a lecithinized delivery system of curcumin (Meriva®) to alleviate the adverse effects of cancer treatment. Phytotherapy Research. 2014 Mar;28(3):444-50; Mansouri K, Rasoulpoor S et al. Clinical effects of curcumin in enhancing cancer therapy: a systematic review. BMC Cancer. 2020;20(1):791.
- Chen WT, Yang TS et al. Effectiveness of a novel herbal agent MB-6 as a potential adjunct to 5-fluoracil-based chemotherapy in colorectal cancer. Nutrition Research. 2014 Jul;34(7):585-94.
- Alsherbiny MA, Abd-Elsalam WH et al. Ameliorative and protective effects of ginger and its main constituents against natural, chemical and radiation-induced toxicities: a comprehensive review. Food and Chemical Toxicology. 2019 Jan;123:72-97; Saxena R, Rida PC, Kucuk O, Aneja R. Ginger augmented chemotherapy: a novel multitarget nontoxic approach for cancer management. Molecular Nutrition & Food Research. 2016 Jun;60(6):1364-73.
- Yeung KS, Gubili J, Mao JJ. Herb-drug interactions in cancer care. Oncology (Williston Park). 2018 Oct 15;32(10):516-20; Marx W, Ried K et al. Ginger—mechanism of action in chemotherapy-induced nausea and ; vomiting: a review. Critical Reviews in Food Science and Nutrition. 2017 Jan 2;57(1):141-146; Marx W, Kiss N, Isenring L. Is ginger beneficial for nausea and vomiting? An update of the literature. Current Opinion in supportive and palliative care. 2015 jun;9(2):189-95; Chen D, Zhao J, Cong W. Chinese herbal medicines facilitate the control of chemotherapy-induced side effects in colorectal cancer: progress and perspective. Frontiers in Pharmacology. 2018 Dec 7;9:1442; Marx W, McCarthy AL et al. The effect of a standardized ginger extract on chemotherapy-induced nausea-related quality of life in patients undergoing moderately or highly emetogenic chemotherapy: a double blind, randomized, placebo controlled trial. Nutrients. 2017 Aug 12;9(8). pii: E867; Ryan JL, Heckler CE et al. Ginger (Zingiber officinale) reduces acute chemotherapy-induced nausea: a URCC CCOP study of 576 patients. Supportive Care in Cancer. 2012 Jul;20(7):1479-89; Zick SM, Ruffin MT et al. Phase II trial of encapsulated ginger as a treatment for chemotherapy-induced nausea and vomiting. Supportive Care in Cancer. 2009 May;17(5):563-72.
- Wang WS, Lin JK et al. Oral glutamine is effective for preventing oxaliplatin-induced neuropathy in colorectal cancer patients. Oncologist. 2007 Mar;12(3):312-9.
- Vahdat L, Papadopoulos K et al. Reduction of paclitaxel-induced peripheral neuropathy with glutamine. Clinical Cancer Research. 2001 May;7(5):1192-7.
- Brami C, Bao T, Deng G. Natural products and complementary therapies for chemotherapy-induced peripheral neuropathy: a systematic review. Critical Reviews in Oncology/Hematology. 2016 Feb;98:325-34.
- Jolfaie NR, Mirzaie S, Ghiasvand R, Askari G, Miraghajani M. The effect of glutamine intake on complications of colorectal and colon cancer treatment: a systematic review. Journal of Research in Medical Sciences. 2015 Sep;20(9):910-8.
- Sun J, Wang H, Hu H. Glutamine for chemotherapy induced diarrhea: a meta-analysis. Asia Pacific Journal of Clinical Nutrition. 2012;21(3):380-5.
- Li Y, Ping X et al. Clinical trial: prophylactic intravenous alanyl-glutamine reduces the severity of gastrointestinal toxicity induced by chemotherapy--a randomized crossover study. Alimentary Pharmacology & Therapeutics. 2009 Sep 1;30(5):452-8.
- Ogata Y, Ishibashi N et al. Preventive effects of amino-acid-rich elemental diet Elental® on chemotherapy-induced oral mucositis in patients with colorectal cancer: a prospective pilot study. Supportive Care in Cancer. 2016 Feb;24(2):783-789.
- Daniele B, Perrone F et al. Oral glutamine in the prevention of fluorouracil induced intestinal toxicity: a double blind, placebo controlled, randomised trial. Gut. 2001 Jan;48(1):28-33.
- Khemissa F, Mineur L et al. A phase III study evaluating oral glutamine and transforming growth factor-beta 2 on chemotherapy-induced toxicity in patients with digestive neoplasm. Digestive and Liver Disease. 2016 Mar;48(3):327-32.
- Kucuktulu E, Guner A, Kahraman I, Topbas M, Kucuktulu U. The protective effects of glutamine on radiation-induced diarrhea. Supportive Care in Cancer. 2013 Apr;21(4):1071-5.
- Rotovnik Kozjek N, Kompan L et al. Oral glutamine supplementation during preoperative radiochemotherapy in patients with rectal cancer: a randomised double blinded, placebo controlled pilot study. Clinical Nutrition. 2011 Oct;30(5):567-70.
- Vidal-Casariego A, Calleja-Fernández A et al. Efficacy of glutamine in the prevention of acute radiation enteritis: a randomized controlled trial. JPEN. Journal of Parenteral and Enteral Nutrition. 2014 Feb;38(2):205-13.
- Block KI, Block PB, Gyllenhaal C. Integrative treatment for colorectal cancer: a comprehensive approach. Journal of Alternative and Complementary Medicine. 2018;24(9-10):890–901.
- Kouhi Habibi N, Shabestani Monfared A et al. The protective effects of melatonin on blood cell counts of rectal cancer patients following radio-chemotherapy: a randomized controlled trial. Clinical and Translational Oncology. 2019;21(6):745-752.
- Lissoni P. Is there a role for melatonin in supportive care? Supportive Care in Cancer. 2002 Mar;10(2):110-6; Lissoni P, Barni S et al. Decreased toxicity and increased efficacy of cancer chemotherapy using the pineal hormone melatonin in metastatic solid tumour patients with poor clinical status. European Journal of Cancer. 1999;35(12):1688-1692; Lissoni P, Tancini G et al. Treatment of cancer chemotherapy-induced toxicity with the pineal hormone melatonin. Supportive Care in Cancer. 1997;5(2):126-129.
- Lissoni P, Brivio F et al. Immune effects of preoperative immunotherapy with high-dose subcutaneous interleukin-2 versus neuroimmunotherapy with low-dose interleukin-2 plus the neurohormone melatonin in gastrointestinal tract tumor patients. Journal of Biological Regulators and Homeostatic Agents. 1995;9(1):31-33.
- de Aguiar Pastore Silva J, de Souza Fabre ME, Waitzberg DL. Omega-3 supplements for patients in chemotherapy and/or radiotherapy: a systematic review. Clinical Nutrition. 2015 Jun;34(3):359-66.
- Silva Jde A, Trindade EB et al. Fish oil supplement alters markers of inflammatory and nutritional status in colorectal cancer patients. Nutrition and Cancer. 2012;64(2):267-73.
- Lavriv DS, Neves PM, Ravasco P. Should omega-3 fatty acids be used for adjuvant treatment of cancer cachexia? Clin Nutr ESPEN. 2018 Jun;25:18-25.
- Ewaschuk JB, Almasud A, Mazurak VC. Role of n-3 fatty acids in muscle loss and myosteatosis. Applied Physiology, Nutrition, and Metabolism. 2014 Jun;39(6):654-62.
- Golkhalkhali B, Rajandram R et al. Strain-specific probiotic (microbial cell preparation) and omega-3 fatty acid in modulating quality of life and inflammatory markers in colorectal cancer patients: a randomized controlled trial. Asia-Pacific Journal of Clinical Oncology. 2018 Jun;14(3):179-191.
- Xie H, Chang YN. Omega-3 polyunsaturated fatty acids in the prevention of postoperative complications in colorectal cancer: a meta-analysis. OncoTargets and Therapy. 2016 Dec 9;9:7435-7443.
- Sorensen LS, Thorlacius-Ussing O et al. Randomized clinical trial of perioperative omega-3 fatty acid supplements in elective colorectal cancer surgery. British Journal of Surgery. 2014 Jan;101(2):33-42.
- Trabal J, Leyes P, Forga M, Maurel J. Potential usefulness of an EPA-enriched nutritional supplement on chemotherapy tolerability in cancer patients without overt malnutrition. Nutricion Hospitalaria. 2010 Sep-Oct;25(5):736-40.
- Read JA, Beale PJ et al. Nutrition intervention using an eicosapentaenoic acid (EPA)-containing supplement in patients with advanced colorectal cancer. Effects on nutritional and inflammatory status: a phase II trial. Supportive Care in Cancer. 2007 Mar;15(3):301-7.
- Pappalardo G, Almeida A, Ravasco P. Eicosapentaenoic acid in cancer improves body composition and modulates metabolism. Nutrition. 2015 Apr;31(4):549-55.
- Wang YH, Yao N et al. The efficacy and safety of probiotics for prevention of chemoradiotherapy-induced diarrhea in people with abdominal and pelvic cancer: a systematic review and meta-analysis. European Journal of Clinical Nutrition. 2016;70(11):1246–1253.
- Lee JY, Chu SH et al. Effects of 12 weeks of probiotic supplementation on quality of life in colorectal cancer survivors: a double-blind, randomized, placebo-controlled trial. Digestive and Liver Disease. 2014;46(12):1126–1132.
- Anderson SW, Bazzell AF, Dains JE. An integrative review on the effect of prebiotics, probiotics, and synbiotics on infection after colorectal cancer surgery. AORN Journal. 2018;107(2):237–248; Aisu N, Tanimura S et al. Impact of perioperative probiotic treatment for surgical site infections in patients with colorectal cancer. Experimental and Therapeutic Medicine. 2015;10(3):966–972.
- Abrams DI. The therapeutic effects of cannabis and cannabinoids: an update from the National Academies of Sciences, Engineering and Medicine report. European Journal of Internal Medicine. 2018 Mar;49:7-11.
- Bar-Lev Schleider L, Mechoulam R et al. Prospective analysis of safety and efficacy of medical cannabis in large unselected population of patients with cancer. European Journal of Internal Medicine. 2018 Mar;49:37-43.
- Häuser W, Welsch P, Klose P, Radbruch L, Fitzcharles MA. Efficacy, tolerability and safety of cannabis-based medicines for cancer pain: a systematic review with meta-analysis of randomised controlled trials. Der Schmerz. 2019 Oct;33(5):424-436.
- Milla P, Airoldi M et al. Administration of reduced glutathione in FOLFOX4 adjuvant treatment for colorectal cancer: effect on oxaliplatin pharmacokinetics, Pt-DNA adduct formation, and neurotoxicity. Anticancer Drugs. 2009 Jun;20(5):396-402.
- Fu X, Wu H et al. Efficacy of drug interventions for chemotherapy-induced chronic peripheral neurotoxicity: a network meta-analysis. Frontiers in Neurology. 2017 Jun 8;8:223.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Serefko A, Szopa A, Poleszak E. Magnesium and depression. Magnesium Research. 2016;29(3):112–119; Cuciureanu MD, Vink R. Magnesium and stress. In Magnesium in the Central Nervous System [Internet]. Adelaide (AU): University of Adelaide Press; 2011.
- Boyle NB, Lawton C, Dye L. The effects of magnesium supplementation on subjective anxiety and stress—a systematic review. Nutrients. 2017;9(5):429.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Bock PR, Hanisch J, Matthes H, Zänker KS. Targeting inflammation in cancer-related-fatigue: a rationale for mistletoe therapy as supportive care in colorectal cancer patients. Inflammation & Allergy Drug Targets. 2014;13(2):105-11.
- Friedel WE, Matthes H, Bock PR, Zänker KS. Systematic evaluation of the clinical effects of supportive mistletoe treatment within chemo- and/or radiotherapy protocols and long-term mistletoe application in nonmetastatic colorectal carcinoma: multicenter, controlled, observational cohort study. Journal of the Society for Integrative Oncology. 2009 Fall;7(4):137-45.
- Thronicke A, Oei SL, Merkle A, Matthes H, Schad F. Clinical safety of combined targeted and Viscum album L. therapy in oncological patients. Medicines (Basel). 2018 Sep 6;5(3). pii: E100.
- Alschuler LN, Gazella KA. The Definitive Guide to Cancer, 3rd Edition: An Integrative Approach to Prevention, Treatment, and Healing. Berkeley, California: Celestial Arts. 2010; Alschuler LN, Gazella KA. The Definitive Guide to Thriving after Cancer: A Five-Step Integrative Plan to Reduce the Risk of Recurrence and Build Lifelong Health. Berkeley, California: Ten Speed Press. 2013.
- McKinney N. Naturopathic Oncology, 3rd Edition. Victoria, BC, Canada: Liaison Press. 2016.
- Parmar G, Kaczor T. Textbook of Naturopathic Oncology: A Desktop Guide of Integrative Cancer Care. 1st edition. Canada: Medicatrix Holdings Ltd. 2020.
- Ali BH. Amelioration of oxaliplatin neurotoxicity by drugs in humans and experimental animals: a minireview of recent literature. Basic & Clinical Pharmacology & Toxicology. 2010;106(4):272–279; Lin PC, Lee MY et al. N-acetylcysteine has neuroprotective effects against oxaliplatin-based adjuvant chemotherapy in colon cancer patients: preliminary data. Supportive Care in Cancer. 2006;14(5):484–487.
- Schloss JM, Colosimo M, Airey C, Masci PP, Linnane AW, Vitetta L. Nutraceuticals and chemotherapy induced peripheral neuropathy (CIPN): a systematic review [published correction appears in Clinical Nutrition. 2015 Feb;34(1):167]. Clinical Nutrition. 2013;32(6):888–893; Albers JW, Chaudhry V, Cavaletti G, Donehower RC. Interventions for preventing neuropathy caused by cisplatin and related compounds. Cochrane Database of Systematic Reviews. 2014;(3):CD005228; Ali BH. Amelioration of oxaliplatin neurotoxicity by drugs in humans and experimental animals: a minireview of recent literature. Basic and Clinical Pharmacology and Toxicology. 2010;106(4):272–279.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Wang QZ, Chen XP, Huang JP, Jiang XW. [Effects of couplet medicines (Astragalus membranaceus and jiaozhen) on intestinal barrier in postoperative colorectal cancer patients] [Article in Chinese]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2015;35(11):1307–1312.
- Grothey A, Nikcevich DA et al. Intravenous calcium and magnesium for oxaliplatin-induced sensory neurotoxicity in adjuvant colon cancer: NCCTG N04C7. Journal of Clinical Oncology. 2011 Feb 1;29(4):421-7.
- Gamelin L, Boisdron-Celle M et al. Prevention of oxaliplatin-related neurotoxicity by calcium and magnesium infusions: a retrospective study of 161 patients receiving oxaliplatin combined with 5-Fluorouracil and leucovorin for advanced colorectal cancer. Clinical Cancer Research. 2004 Jun 15;10(12 Pt 1):4055-61.
- Wu Z, Ouyang J, He Z, Zhang S. Infusion of calcium and magnesium for oxaliplatin-induced sensory neurotoxicity in colorectal cancer: a systematic review and meta-analysis. European Journal of Cancer. 2012 Aug;48(12):1791-8.
- Huang S, Peng W et al. Kangai injection, a traditional Chinese medicine, improves efficacy and reduces toxicity of chemotherapy in advanced colorectal cancer patients: a systematic review and meta-analysis. Evidence-based Complementary and Alternative Medicine. 2019 Jul 15;2019:8423037.
- Yu R, Wu X, Jia L, Lou Y. Effect of Chinese herbal compound LC09 on patients with capecitabine-associated hand-foot syndrome: a randomized, double-blind, and parallel-controlled trial. Integrative Cancer Therapies. Jan-Dec 2020;19:1534735420928466.
- Mantovani G, Macciò A et al. Randomized phase III clinical trial of five different arms of treatment in 332 patients with cancer cachexia. Oncologist. 2010;15(2):200-11.
- Yang YF, Xu Y, Wu Y. [Clinical randomized double-blinded controlled study on Quxie Capsule in reducing post-operational relapse and metastasis of colorectal cancer]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2007;27(10):879–882.
- Lagoa R, Silva J, Rodrigues JR, Bishayee A. Advances in phytochemical delivery systems for improved anticancer activity. Biotechnology Advances. 2019 Apr 9. pii: S0734-9750(19)30063-1; Amalraj A, Pius A, Gopi S, Gopi S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—a review. Journal of Traditional and Complementary Medicine. 2016 Jun 15;7(2):205-233; Belcaro G, Hosoi M et al. A controlled study of a lecithinized delivery system of curcumin (Meriva®) to alleviate the adverse effects of cancer treatment. Phytotherapy Research. 2014 Mar;28(3):444-50.
- Amalraj A, Pius A, Gopi S, Gopi S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—a review. Journal of Traditional and Complementary Medicine. 2016 Jun 15;7(2):205-233.
- Panahi Y, Saadat A, Beiraghdar F, Sahebkar A. Adjuvant therapy with bioavailability-boosted curcuminoids suppresses systemic inflammation and improves quality of life in patients with solid tumors: a randomized double-blind placebo-controlled trial. Phytotherapy Research. 2014 Oct;28(10):1461-7.
- Yeung KS, Gubili J, Mao JJ. Herb-drug interactions in cancer care. Oncology (Williston Park). 2018 Oct 15;32(10):516-20; Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS Journal. 2013 Jan;15(1):195-218.
- Yeung KS, Gubili J, Mao JJ. Herb-drug interactions in cancer care. Oncology (Williston Park). 2018 Oct 15;32(10):516-20.
- Amalraj A, Pius A, Gopi S, Gopi S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—a review. Journal of Traditional and Complementary Medicine. 2016 Jun 15;7(2):205-233.
- Chen D, Zhao J, Cong W. Chinese herbal medicines facilitate the control of chemotherapy-induced side effects in colorectal cancer: progress and perspective. Frontiers in Pharmacology. 2018 Dec 7;9:1442.
- Mueller T, Voigt W. Fermented wheat germ extract—nutritional supplement or anticancer drug? Nutrition Journal. 2011 Sep 5;10:89; Yeend T, Robinson K, Lockwood C, McArthur A. The effectiveness of fermented wheat germ extract as an adjunct therapy in the treatment of cancer: a systematic review. JBI Library of Systematic Reviews. 2012;10(42 Suppl):1-12.
- Yeend T, Robinson K, Lockwood C, McArthur A. The effectiveness of fermented wheat germ extract as an adjunct therapy in the treatment of cancer: a systematic review. JBI Library of Systematic Reviews. 2012;10(42 Suppl):1-12.
- Yehia R, Saleh S, El Abhar H, Saad AS, Schaalan M. L-Carnosine protects against oxaliplatin-induced peripheral neuropathy in colorectal cancer patients: a perspective on targeting Nrf-2 and NF-κB pathways. Toxicology and Applied Pharmacology. 2019;365:41-50.
- Harvie M. Nutritional supplements and cancer: potential benefits and proven harms. American Society of Clinical Oncology Educational Book. 2014;e478-e486.
- Schloss J, Colosimo M. B vitamin complex and chemotherapy-induced peripheral neuropathy. Current Oncology Reports. 2017;19(12):76; Schloss JM, Colosimo M et al. A randomised, placebo-controlled trial assessing the efficacy of an oral B group vitamin in preventing the development of chemotherapy-induced peripheral neuropathy (CIPN). Supportive Care in Cancer. 2017;25(1):195–204; Rostock M, Jaroslawski K et al. Chemotherapy-induced peripheral neuropathy in cancer patients: a four-arm randomized trial on the effectiveness of electroacupuncture. Evidence-Based Complementary and Alternative Medicine. 2013;2013:349653; Schloss JM, Colosimo M, Airey C, Masci PP, Linnane AW, Vitetta L. Nutraceuticals and chemotherapy induced peripheral neuropathy (CIPN): a systematic review [published correction appears in Clinical Nutrition. 2015 Feb;34(1):167]. Clinical Nutrition. 2013;32(6):888–893; Kim PY, Johnson CE. Chemotherapy-induced peripheral neuropathy: a review of recent findings. Current Opinion in Anesthesiology. 2017;30(5):570–576.
- Zhang M, Han W, Hu S, Xu H. Methylcobalamin: a potential vitamin of pain killer. Neural Plasticity. 2013;2013:424651.
- Yeom CH, Jung GC, Song KJ. Changes of terminal cancer patients' health-related quality of life after high dose vitamin C administration. Journal of Korean Medical Science. 2007 Feb;22(1):7-11.
- Takahashi H, Mizuno Haruyoshi Yanagisawa A. High-dose intravenous vitamin C improves quality of life in cancer patients. Personalized Medicine Universe. 2012 Jul;1(1):49-53.
- El Halabi I, Bejjany R et al. Ascorbic acid in colon cancer: from the basic to the clinical applications. International Journal of Moleculal Sciences. 2018;19(9):2752.
- Harvie M. Nutritional supplements and cancer: potential benefits and proven harms. American Society of Clinical Oncology Educational Book. 2014;e478-e486.
- Harvie M. Nutritional supplements and cancer: potential benefits and proven harms. American Society of Clinical Oncology Educational Book. 2014;e478-e486.
- Horie T, Matsumoto H et al. Protective effect of aged garlic extract on the small intestinal damage of rats induced by methotrexate administration. Planta Medica. 1999 Aug;65(6):545-8; Horie T, Awazu S, Itakura Y, Fuwa T. Alleviation by garlic of antitumor drug-induced damage to the intestine. Journal of Nutrition. 2001 Mar;131(3s):1071S-4S.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Cheah KY, Howarth GS, Bastian SE. Grape seed extract dose-responsively decreases disease severity in a rat model of mucositis; concomitantly enhancing chemotherapeutic effectiveness in colon cancer cells. PLoS One. 2014 Jan 21;9(1):e85184.
- Zhong Z, Wheeler MD et al. L-glycine: a novel antiinflammatory, immunomodulatory, and cytoprotective agent. Current Opinions in Clinical Nutrition and Metabolic Care. 2003 Mar;6(2):229-40; Mikalauskas S, Mikalauskiene L et al. Dietary glycine protects from chemotherapy-induced hepatotoxicity. Amino Acids. 2011 Apr;40(4):1139-50.
- Elliott WJ. Timing treatment to the rhythm of disease: a short course in chronotherapeutics. Postgraduate Medicine. 2001 Aug;110(2):119-22, 125-6, 129; Lévi F, Zidani R, Misset JL. Randomised multicentre trial of chronotherapy with oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer. International Organization for Cancer Chronotherapy. Lancet. 1997 Sep 6;350(9079):681-6.
- Lévi F, Zidani R, Misset JL. Randomised multicentre trial of chronotherapy with oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer. International Organization for Cancer Chronotherapy. Lancet. 1997 Sep 6;350(9079):681-6.
- Lévi FA, Zidani R et al. Chronomodulated versus fixed-infusion-rate delivery of ambulatory chemotherapy with oxaliplatin, fluorouracil, and folinic acid (leucovorin) in patients with colorectal cancer metastases: a randomized multi-institutional trial. Journal of the National Cancer Institute. 1994 Nov 2;86(21):1608-17.
- Baker D. Application of chronotherapy to the treatment of cancer: can changing the timing of drug administration influence efficacy and toxicity? Advances in Pharmacy. 2004 Jul;2(3):222-228.
- Mormont MC, Waterhouse J et al. Marked 24-h rest/activity rhythms are associated with better quality of life, better response, and longer survival in patients with metastatic colorectal cancer and good performance status. Clinical Cancer Research. 2000;6(8):3038–3045.
- Lévi F, Focan C et al. Implications of circadian clocks for the rhythmic delivery of cancer therapeutics. Advanced Drug Delivery Reviews. 2007;59(9-10):1015–1035.
- Boughattas NA, Lévi F et al. Stable circadian mechanisms of toxicity of two platinum analogs (cisplatin and carboplatin) despite repeated dosages in mice. Journal of Pharmacology and Experimental Therapeutics. 1990 Nov;255(2):672-9.
- El-Fatatry BM, Ibrahim OM, Hussien FZ, Mostafa TM. Role of metformin in oxaliplatin-induced peripheral neuropathy in patients with stage III colorectal cancer: randomized, controlled study. International Journal of Colorectal Disease. 2018 Dec;33(12):1675-1683.
- Patterson S. Metformin may have broad utility in cancer. MD Anderson Cancer Center. November-December 2014. Viewed January 19, 2018.
- Zhou W, Kavelaars A, Heijnen CJ. Metformin prevents cisplatin-induced cognitive impairment and brain damage in mice. PLoS ONE. 2016;11(3):e0151890.
- Elwood PC, Morgan G et al. Aspirin in the treatment of cancer: reductions in metastatic spread and in mortality: a systematic review and meta-analyses of published studies. PLoS One. 2016 Apr 20;11(4):e0152402.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Choi J, Lee EJ, Yang SH, Im YR, Seong J. A prospective Phase II study for the efficacy of radiotherapy in combination with zoledronic acid in treating painful bone metastases from gastrointestinal cancers. Journal of Radiation Research. 2019;60(2):242–248.
- Pantziarka P, Bouche G, Meheus L, Sukhatme V, Sukhatme VP. Repurposing drugs in oncology (ReDO)—cimetidine as an anti-cancer agent. Ecancermedicalscience. 2014;8:485.
- Pantziarka P, Bouche G, Meheus L, Sukhatme V, Sukhatme VP. Repurposing drugs in oncology (ReDO)—cimetidine as an anti-cancer agent. Ecancermedicalscience. 2014;8:485.
- Qu B, Qu H. The influence of statins on risk and patient survival in colorectal cancer. Journal of Clinical Gastroenterology. 2019;53(9):699–701.
- Block KI, Block PB, Gyllenhaal C. Integrative treatment for colorectal cancer: a comprehensive approach. Journal of Alternative and Complementary Medicine. 2018;24(9-10):890–901.
- Safdie FM, Dorff T, Quinn D, et al. Fasting and cancer treatment in humans: a case series report. Aging (Albany NY). 2009;1(12):988–1007.
- Dorff TB, Groshen S et al. Safety and feasibility of fasting in combination with platinum-based chemotherapy. BMC Cancer. 2016;16:360.
- Lévesque S, Pol JG, Ferrere G, Galluzzi L, Zitvogel L, Kroemer G. Trial watch: dietary interventions for cancer therapy. Oncoimmunology. 2019;8(7):1591878.
- Dorff TB, Groshen S et al. Safety and feasibility of fasting in combination with platinum-based chemotherapy. BMC Cancer. 2016;16:360.
- Jongbloed F, Huisman SA, van Steeg H, et al. The transcriptomic response to irinotecan in colon carcinoma bearing mice preconditioned by fasting. Oncotarget. 2019;10(22):2224–2234.
- Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Research Reviews. 2017;39:46–58; Nencioni A, Caffa I, Cortellino S, Longo VD. Fasting and cancer: molecular mechanisms and clinical application. Nature Reviews. Cancer 2018;18(11):707–719.
- Cheng CW, Adams Gregor B et al. Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosuppression. Cell Stem Cell. 2014;14:810–823.
- Block KI, Block PB, Gyllenhaal C. Integrative treatment for colorectal cancer: a comprehensive approach. Journal of Alternative and Complementary Medicine. 2018;24(9-10):890–901.
- Tusek DL, Church JM, Strong SA, Grass JA, Fazio VW. Guided imagery: a significant advance in the care of patients undergoing elective colorectal surgery. Diseases of the Colon and Rectum. 1997 Feb;40(2):172-8.
- Derksen TM, Bours MJ, Mols F, Weijenberg MP. Lifestyle-related factors in the self-management of chemotherapy-induced peripheral neuropathy in colorectal cancer: a systematic review. Evidence-Based Complementary and Alternative Medicine. 2017;2017:7916031.
- Chien A, Yang CC et al. Ultrasound acupuncture for oxaliplatin-induced peripheral neuropathy in patients with colorectal cancer: a pilot study. PM&R. 2020;10.1002/pmrj.12361.
- Chan K, Lui L et al. The efficacy and safety of electro-acupuncture for alleviating chemotherapy-induced peripheral neuropathy in patients with colorectal cancer: study protocol for a single-blinded, randomized sham-controlled trial. Trials. 2020;21(1):58.
- Gu XY, Gao ZQ, Zhang ZJ, Huang ZM, Xie XH. [Influence of warming needling technique on gastrointestinal reaction after hyperthermic intrape-ritoneal chemotherapy in patients with postoperation of colon cancer] [Article in Chinese]. Zhen Ci Yan Jiu. 2020;45(4):315-319.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Care. New York: Bantam Dell. 2009; Ning Y, Wang L, Giovannucci EL. A quantitative analysis of body mass index and colorectal cancer: findings from 56 observational studies. Obesity Reviews. 2010 Jan;11(1):19-30; Bardou M, Barkun AN, Martel M. Obesity and colorectal cancer. Gut. 2013 Jun;62(6):933-47; Shaukat A, Dostal A, Menk J, Church TR. BMI is a risk factor for colorectal cancer mortality. Digestive Diseases and Sciences. 2017 Sep;62(9):2511-2517; Lee J, Jeon JY, Meyerhardt JA. Diet and lifestyle in survivors of colorectal cancer. Hematology/Oncology Clinics of North America. 2015 Feb;29(1):1-27; National Cancer Institute. Colorectal Cancer Prevention (PDQ®)–Patient Version. March 15, 2019. Viewed May 14, 2019; Thanikachalam K, Khan G. Colorectal cancer and nutrition. Nutrients. 2019 Jan 14;11(1). pii: E164; Brand MP, Peeters PH, van Gils CH, Elias SG. Pre-adult famine exposure and subsequent colorectal cancer risk in women. International Journal of Epidemiology. 2017 Apr 1;46(2):612-621; Qu B, Qu H. The influence of statins on risk and patient survival in colorectal cancer. Journal of Clinical Gastroenterology. 2019;53(9):699–701; Ali Khan U, Fallah M et al. Personal history of diabetes as important as family history of colorectal cancer for risk of colorectal cancer: a nationwide cohort study. American Journal of Gastroenterology. 2020;10.14309/ajg.0000000000000669.
- Mayo Clinic Staff. Metabolic syndrome. Mayo Clinic. Viewed February 22, 2021.
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Diet, nutrition, physical activity and colorectal cancer. Viewed May 14, 2019; American Cancer Society: Can Colorectal Cancer Be Prevented? May 30, 2018. Viewed May 14, 2019; National Cancer Institute: Colorectal Cancer Prevention (PDQ®)–Patient Version. March 15, 2019. Viewed May 14, 2019; American Institute for Cancer Research. Cancer Prevention Recommendations. Viewed May 14, 2019; Wang X, Ji A et al. A meta-analysis including dose-response relationship between night shift work and the risk of colorectal cancer. Oncotarget. 2015 Sep 22;6(28):25046-60; Parent ME, El-Zein M, Rousseau M-C, Pintos J, Siemiatycki J. Night work and the risk of cancer among men. American Journal of Epidemiology. 2012 Nov 1;176(9):751-9; Hrushesky WJ, Lannin D, Haus E. Evidence for an ontogenetic basis for circadian coordination of cancer cell proliferation. Journal of the National Cancer Institute. 1998 Oct 7;90(19):1480-4; Durko L, Malecka-Panas E et al. Lifestyle modifications and colorectal cancer. Current Colorectal Cancer Reports. 2014;10:45-54; Garcia H, Song M. Early-life obesity and adulthood colorectal cancer risk: a meta-analysis. Revista Panamericana de Salud Publica. 2019 Jan 4;43:e3; Thanikachalam K, Khan G. Colorectal cancer and nutrition. Nutrients. 2019 Jan 14;11(1). pii: E164; Vainio H, Kaaks R, Bianchini F. Weight control and physical activity in cancer prevention: international evaluation of the evidence. European Journal of Cancer Prevention. 2002 Aug;11 Suppl 2:S94-100; Bailie L, Loughrey MB, Coleman HG. Lifestyle risk factors for serrated colorectal polyps: a systematic review and meta-analysis. Gastroenterology. 2017 Jan;152(1):92-104; Song M, Chan AT. Environmental factors, gut microbiota, and colorectal cancer prevention. Clinical Gastroenterology and Hepatology. 2019 Jan;17(2):275-289; Qu B, Qu H. The influence of statins on risk and patient survival in colorectal cancer. Journal of Clinical Gastroenterology. 2019;53(9):699–701; Dolejs SC, Gayed B, Fajardo A. Prevention of colorectal neoplasia. Clinics in Colon and Rectal Surgery. 2016;29(4):353-362; Derry MM, Raina K, Agarwal C, Agarwal R. Identifying molecular targets of lifestyle modifications in colon cancer prevention. Frontiers in Oncology. 2013 May 14;3:119; Kuipers EJ, Grady WM et al. Colorectal cancer. Nature Reviews. Disease Primers. 2015;1:15065; Chapelle N, Martel M, Toes-Zoutendijk E, Barkun AN, Bardou M. Recent advances in clinical practice: colorectal cancer chemoprevention in the average-risk population. Gut. 2020 Sep 28:gutjnl-2020-320990.
- Arthur RS, Dannenberg AJ, Kim M, Rohan TE. The association of body fat composition with risk of breast, endometrial, ovarian and colorectal cancers among normal weight participants in the UK Biobank. British Journal of Cancer. 2021 Mar 15.
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Diet, nutrition, physical activity and colorectal cancer. Viewed October 5, 2020.
- Keum N, Aune D, Greenwood DC, Ju W, Giovannucci EL. Calcium intake and colorectal cancer risk: dose-response meta-analysis of prospective observational studies. International Journal of Cancer. 2014 Oct 15;135(8):1940-8. Dolejs SC, Gayed B, Fajardo A. Prevention of colorectal neoplasia. Clinics in Colon and Rectal Surgery. 2016;29(4):353-362.
- Thanikachalam K, Khan G. Colorectal cancer and nutrition. Nutrients. 2019 Jan 14;11(1). pii: E164; Cauley JA, Chlebowski RT et al. Calcium plus vitamin D supplementation and health outcomes five years after active intervention ended: the Women's Health Initiative. Journal of Women’s Health (Larchmt). 2013 Nov;22(11):915-29; Katona BW, Weiss JM. Chemoprevention of colorectal cancer. Gastroenterology. 2020;158(2):368–388.
- Chen GC, Pang Z, Liu QF. Magnesium intake and risk of colorectal cancer: a meta-analysis of prospective studies. European Journal of Clinical Nutrition. 2012 Nov;66(11):1182-6; Chapelle N, Martel M, Toes-Zoutendijk E, Barkun AN, Bardou M. Recent advances in clinical practice: colorectal cancer chemoprevention in the average-risk population. Gut. 2020 Dec;69(12):2244-2255.
- Ohwada S, Ikeya T et al. Adjuvant immunochemotherapy with oral Tegafur/Uracil plus PSK in patients with stage II or III colorectal cancer: a randomised controlled study. British Journal of Cancer. 2004 Mar 8;90(5):1003-10.
- Oka S, Tanaka S et al. A water-soluble extract from culture medium of Ganoderma lucidum mycelia suppresses the development of colorectal adenomas. Hiroshima Journal of Medical Sciences. 59 (1): 1-6, 2010.
- Xie JT, Wang CZ et al. Ganoderma lucidum extract inhibits proliferation of SW 480 human colorectal cancer cells. Experimental Oncology. 2006 Mar;28(1):25-9.
- Ben S, Du M et al. Vitamin B2 intake reduces the risk for colorectal cancer: a dose-response analysis. European Journal of Nutrition. 2019;58(4):1591–1602.
- Thanikachalam K, Khan G. Colorectal cancer and nutrition. Nutrients. 2019 Jan 14;11(1). pii: E164; Larsson SC, Orsini N, Wolk A. Vitamin B6 and risk of colorectal cancer: a meta-analysis of prospective studies. JAMA. 2010 Mar 17;303(11):1077-83.
- Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS Journal . 2013 Jan;15(1):195-218; Carroll RE, Benya RV et al. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prevention Research (Philadelphia, Pennsylvania). 2011 Mar;4(3):354-64; Fadus MC, Lau C, Bikhchandani J, Lynch HT. Curcumin: an age-old anti-inflammatory and anti-neoplastic agent. Journal of Traditional and Complementary Medicine. 2016 Sep 9;7(3):339-346; Cruz-Correa M, Shoskes DA, Sanchez P, Zhao R, Hylind LM, Wexner SD, Giardiello FM. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clinical Gastroenterology and Hepatology. 2006 Aug;4(8):1035-8. .
- Gulbake A, Jain A, Jain A, Jain A, Jain SK. Insight to drug delivery aspects for colorectal cancer. World Journal of Gastroenterology. 2016 Jan 14;22(2):582-99.
- Alschuler LN, Gazella KA. The Definitive Guide to Cancer, 3rd Edition: An Integrative Approach to Prevention, Treatment, and Healing. Berkeley, California: Celestial Arts. 2010; Alschuler LN, Gazella KA. The Definitive Guide to Thriving after Cancer: A Five-Step Integrative Plan to Reduce the Risk of Recurrence and Build Lifelong Health. Berkeley, California: Ten Speed Press. 2013.
- Bar-Sela G, Epelbaum R, Schaffer M. Curcumin as an anti-cancer agent: review of the gap between basic and clinical applications. Current Medicinal Chemistry. 2010;17(3):190-7.
- Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS Journal. 2013 Jan;15(1):195-218; Carroll RE, Benya RV et al. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prevention Research (Philadelphia, Pennsylvania). 2011 Mar;4(3):354-64.
- Amalraj A, Pius A, Gopi S, Gopi S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—a review. Journal of Traditional and Complementary Medicine. 2016 Jun 15;7(2):205-233.
- Núñez-Sánchez MA, González-Sarrías A et al. Dietary phenolics against colorectal cancer—from promising preclinical results to poor translation into clinical trials: pitfalls and future needs. Molecular Nutrition & Food Research. 2015 Jul;59(7):1274-91.
- Fadus MC, Lau C, Bikhchandani J, Lynch HT. Curcumin: an age-old anti-inflammatory and anti-neoplastic agent. Journal of Traditional and Complementary Medicine. 2016 Sep 9;7(3):339-346; Boral D, Nie D. Cancer stem cells and niche mircoenvironments. Frontiers in Bioscience (Elite Edition). 2012 Jun 1;4:2502-14.
- Hou TY, Davidson LA et al. Nutrient-gene interaction in colon cancer, from the membrane to cellular physiology. Annual Review of Nutrition. 2016 Jul 17;36:543-70.
- Alschuler LN, Gazella KA. The Definitive Guide to Cancer, 3rd Edition: An Integrative Approach to Prevention, Treatment, and Healing. Berkeley, California: Celestial Arts. 2010; Alschuler LN, Gazella KA. The Definitive Guide to Thriving after Cancer: A Five-Step Integrative Plan to Reduce the Risk of Recurrence and Build Lifelong Health. Berkeley, California: Ten Speed Press. 2013.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- McKinney N. Naturopathic Oncology, 3rd Edition. Victoria, BC, Canada: Liaison Press. 2016.
- Liu C, Li P et al. Advances in the antagonism of epigallocatechin-3-gallate in the treatment of digestive tract tumors. Molecules. 2019 May 3;24(9). pii: E1726; Kumar N, Shibata D, Helm J, Coppola D, Malafa M. Green tea polyphenols in the prevention of colon cancer. Frontiers in Bioscience. 2007 Jan 1;12:2309-15; Liu C, Li P et al. Advances in the antagonism of epigallocatechin-3-gallate in the treatment of digestive tract tumors. Molecules. 2019 May 3;24(9). pii: E1726.
- Shimizu M, Adachi S, Masuda M, Kozawa O, Moriwaki H. Cancer chemoprevention with green tea catechins by targeting receptor tyrosine kinases. Molecular Nutrition & Food Research. 2011 Jun;55(6):832-43.
- Shimizu M, Fukutomi Y et al. Green tea extracts for the prevention of metachronous colorectal adenomas: a pilot study. Cancer Epidemiology, Biomarkers & Prevention. 2008 Nov;17(11):3020-5; Shin CM, Lee DH et al. Green tea extracts for the prevention of metachronous colorectal polyps among patients who underwent endoscopic removal of colorectal adenomas: a randomized clinical trial. Clinical Nutrition. 2018 Apr;37(2):452-458.
- Chen J, Huang XF. The signal pathways in azoxymethane-induced colon cancer and preventive implications. Cancer Biology & Therapy. 2009 Jul;8(14):1313-7.
- Fajardo AM, Piazza GA. Chemoprevention in gastrointestinal physiology and disease. Anti-inflammatory approaches for colorectal cancer chemoprevention. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2015 Jul 15;309(2):G59-70; Ullah MF, Bhat SH et al. Pharmacological intervention through dietary nutraceuticals in gastrointestinal neoplasia. Critical Reviews in Food Science and Nutrition. 2016 Jul 3;56(9):1501-18.
- Ullah MF, Bhat SH et al. Pharmacological intervention through dietary nutraceuticals in gastrointestinal neoplasia. Critical Reviews in Food Science and Nutrition. 2016 Jul 3;56(9):1501-18.
- Ullah MF, Bhat SH et al. Pharmacological intervention through dietary nutraceuticals in gastrointestinal neoplasia. Critical Reviews in Food Science and Nutrition. 2016 Jul 3;56(9):1501-18.
- Hu G, Zhang L, Rong Y, Ni X, Sun Y. Downstream carcinogenesis signaling pathways by green tea polyphenols: a translational perspective of chemoprevention and treatment for cancers. Current Drug Metabolism. 2014 Jan;15(1):14-22.
- Alschuler LN, Gazella KA. The Definitive Guide to Cancer, 3rd Edition: An Integrative Approach to Prevention, Treatment, and Healing. Berkeley, California: Celestial Arts. 2010; Alschuler LN, Gazella KA. The Definitive Guide to Thriving after Cancer: A Five-Step Integrative Plan to Reduce the Risk of Recurrence and Build Lifelong Health. Berkeley, California: Ten Speed Press. 2013.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Lemole G, Mehta P, McKee D. After Cancer Care: The Definitive Self-Care Guide to Getting and Staying Well for Patients with Cancer. New York, New York: Rodale, Inc. 2015.
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Diet, nutrition, physical activity and colorectal cancer. Viewed May 14, 2019.
- Zhang SM, Moore SC et al. Folate, vitamin B6, multivitamin supplements, and colorectal cancer risk in women. American Journal of Epidemiology. 2006;163(2):108–115.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Kim S, Sandler DP, Galanko J, Martin C, Sandler RS. Intake of polyunsaturated fatty acids and distal large bowel cancer risk in whites and African Americans. American Journal of Epidemiology. 2010 May 1;171(9):969-79; Satia JA, Littman A, Slatore CG, Galanko JA, White E.Associations of herbal and specialty supplements with lung and colorectal cancer risk in the VITamins and Lifestyle study. Cancer Epidemiology, Biomarkers & Prevention. 2009 May;18(5):1419-28.
- Kantor ED, Lampe JW, Peters U, Vaughan TL, White E. Long-chain omega-3 polyunsaturated fatty acid intake and risk of colorectal cancer. Nutrition and Cancer. 2014;66(4):716-27.
- Katona BW, Weiss JM. Chemoprevention of colorectal cancer. Gastroenterology. 2020;158(2):368–388.
- Zárate R, El Jaber-Vazdekis N, Tejera N, Pérez JA, Rodríguez C. Significance of long chain polyunsaturated fatty acids in human health. Clinical and Translational Medicine. 2017 Dec;6(1):25.
- Song M, Chan AT. Environmental factors, gut microbiota, and colorectal cancer prevention. Clinical Gastroenterology and Hepatology. 2019 Jan;17(2):275-289.
- Hull MA, Sprange K et al. Eicosapentaenoic acid and aspirin, alone and in combination, for the prevention of colorectal adenomas (seAFOod Polyp Prevention trial): a multicentre, randomised, double-blind, placebo-controlled, 2 × 2 factorial trial. Lancet. 2018 Dec 15;392(10164):2583-2594.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Lemole G, Mehta P, McKee D. After Cancer Care: The Definitive Self-Care Guide to Getting and Staying Well for Patients with Cancer. New York, New York: Rodale, Inc. 2015.
- McKinney N. Naturopathic Oncology, 3rd Edition. Victoria, BC, Canada: Liaison Press. 2016.
- Hendler R, Zhang Y. Probiotics in the treatment of colorectal cancer. Medicines (Basel). 2018 Sep 7;5(3):101.
- Lamichhane P, Maiolini M et al. Colorectal cancer and probiotics: Are bugs really drugs? Cancers (Basel). 2020 May 5;12(5):1162; Drago L. Probiotics and colon cancer. Microorganisms. 2019 Feb 28;7(3):66.
- Zheng X, Wu K et al. Yogurt consumption and risk of conventional and serrated precursors of colorectal cancer. Gut. 2019;gutjnl-2019-318374.
- Jacouton E, Chain F, Sokol H, Langella P, Bermúdez-Humarán LG. Probiotic strain Lactobacillus casei BL23 prevents colitis-associated colorectal cancer. Frontiers in Immunology. 2017 Nov 17;8:1553.
- Gao C, Ganesh BP et al. Gut microbe-mediated suppression of inflammation-associated colon carcinogenesis by luminal histamine production. American Journal of Pathology. 2017 Oct;187(10):2323-2336.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- McKinney N. Naturopathic Oncology, 3rd Edition. Victoria, BC, Canada: Liaison Press. 2016.
- Nguyen AV, Martinez M et al. Results of a phase I pilot clinical trial examining the effect of plant-derived resveratrol and grape powder on Wnt pathway target gene expression in colonic mucosa and colon cancer. Cancer Management and Research. 2009 Apr 3;1:25-37.
- Alschuler LN, Gazella KA. The Definitive Guide to Cancer, 3rd Edition: An Integrative Approach to Prevention, Treatment, and Healing. Berkeley, California: Celestial Arts. 2010; Alschuler LN, Gazella KA. The Definitive Guide to Thriving after Cancer: A Five-Step Integrative Plan to Reduce the Risk of Recurrence and Build Lifelong Health. Berkeley, California: Ten Speed Press. 2013.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Crosara Teixeira M, Braghiroli MI, Sabbaga J, Hoff PM. Primary prevention of colorectal cancer: myth or reality? World Journal of Gastroenterology. 2014 Nov 7;20(41):15060-9; Song M, Chan AT. Environmental factors, gut microbiota, and colorectal cancer prevention. Clinical Gastroenterology and Hepatology. 2019 Jan;17(2):275-289; World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Diet, nutrition, physical activity and colorectal cancer. Viewed May 14, 2019; Thanikachalam K, Khan G. Colorectal cancer and nutrition. Nutrients. 2019 Jan 14;11(1). pii: E164; Shang M, Sun J. Vitamin D/VDR, probiotics, and gastrointestinal diseases. Current Medicinal Chemistry. 2017;24(9):876-887; Feskanich D, Ma J et al. Plasma vitamin D metabolites and risk of colorectal cancer in women. Cancer Epidemiology, Biomarkers & Prevention. 2004 Sep;13(9):1502-8; Lee JE, Li H et al. Circulating levels of vitamin D and colon and rectal cancer: the Physicians' Health Study and a meta-analysis of prospective studies. Cancer Prevention Research (Philadelphia). 2011 May;4(5):735-43; McCullough ML, Zoltick ES et al. Circulating vitamin D and colorectal cancer risk: an international pooling project of 17 cohorts. JNCI: Journal of the National Cancer Institute. 2019 Feb 1;111(2):158-169.
- Krstic MN, Mijac DD, Popovic DD, Pavlovic Markovic A, Milosavljević T. General aspects of primary cancer prevention. Digestive Diseases. 2019;37(5):406–415.
- Grau MV, Baron JA et al. Vitamin D, calcium supplementation, and colorectal adenomas: results of a randomized trial. JNCI: Journal of the National Cancer Institute. 2003;95(23):1765-1771.
- Chapelle N, Martel M, Toes-Zoutendijk E, Barkun AN, Bardou M. Recent advances in clinical practice: colorectal cancer chemoprevention in the average-risk population. Gut. 2020 Sep 28:gutjnl-2020-320990; Touvier M, Chan DS et alT. Meta-analyses of vitamin D intake, 25-hydroxyvitamin D status, vitamin D receptor polymorphisms, and colorectal cancer risk. Cancer Epidemiology, Biomarkers & Prevention. 2011 May;20(5):1003-16.
- Krstic MN, Mijac DD, Popovic DD, Pavlovic Markovic A, Milosavljević T. General aspects of primary cancer prevention. Digestive Diseases. 2019;37(5):406–415.
- Katona BW, Weiss JM. Chemoprevention of colorectal cancer. Gastroenterology. 2020;158(2):368–388.
- Ding EL, Mehta S, Fawzi WW, Giovannucci EL. Interaction of estrogen therapy with calcium and vitamin D supplementation on colorectal cancer risk: reanalysis of Women's Health Initiative randomized trial. International Journal of Cancer. 2008 Apr 15;122(8):1690-4.
- Beaty MM, Lee EY, Glauert HP. Influence of dietary calcium and vitamin D on colon epithelial cell proliferation and 1,2-dimethylhydrazine-induced colon carcinogenesis in rats fed high fat diets. Journal of Nutrition. 1993;123(1):144-152.
- Katona BW, Weiss JM. Chemoprevention of colorectal cancer. Gastroenterology. 2020;158(2):368–388.
- Alschuler LN, Gazella KA. The Definitive Guide to Cancer, 3rd Edition: An Integrative Approach to Prevention, Treatment, and Healing. Berkeley, California: Celestial Arts. 2010; Alschuler LN, Gazella KA. The Definitive Guide to Thriving after Cancer: A Five-Step Integrative Plan to Reduce the Risk of Recurrence and Build Lifelong Health. Berkeley, California: Ten Speed Press. 2013.
- Dong Y, Liu Y et al. Link between risk of colorectal cancer and serum vitamin E levels: a meta-analysis of case-control studies. Medicine (Baltimore). 2017 Jul;96(27):e7470.
- Ju J, Picinich SC, et al. Cancer-preventive activities of tocopherols and tocotrienols. Carcinogenesis. 2010;31(4):533-542; Yang CS, Suh N, Kong AN. Does vitamin E prevent or promote cancer? Cancer Prevention Research (Philadelphia). 2012;5(5):701-705.
- Campbell S, Stone W, Whaley S, Krishnan K. Development of gamma (gamma)-tocopherol as a colorectal cancer chemopreventive agent. Critical Reviews in Oncology/Hematology. 2003;47(3):249-259.
- Stone WL, Krishnan K, Campbell SE, Palau VE. The role of antioxidants and pro-oxidants in colon cancer. World Journal of Gastrointestinal Oncology. 2014 Mar 15;6(3):55-66.
- Lippman SM, Klein EA et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2009 Jan 7;301(1):39-51.
- Papaioannou D, Cooper KL et al. Antioxidants in the chemoprevention of colorectal cancer and colorectal adenomas in the general population: a systematic review and meta-analysis. Colorectal Disease. 2011 Oct;13(10):1085-99; Arain MA, Abdul Qadeer A. Systematic review on "vitamin E and prevention of colorectal cancer". Pakistan Journal of Pharmaceutical Sciences. 2010 Apr;23(2):125-30; Harvie M. Nutritional supplements and cancer: potential benefits and proven harms. American Society of Clinical Oncology Educational Book. 2014;e478-e486.
- Lance P, Alberts DS et al. Colorectal adenomas in participants of the SELECT randomized trial of selenium and vitamin E for prostate cancer prevention. Cancer Prevention Research (Philadelphia). 2017 Jan;10(1):45-54.
- Lemole G, Mehta P, McKee D. After Cancer Care: The Definitive Self-Care Guide to Getting and Staying Well for Patients with Cancer. New York, New York: Rodale, Inc. 2015.
- McKinney N. Naturopathic Oncology, 3rd Edition. Victoria, BC, Canada: Liaison Press. 2016.
- Boros LG, Nichelatti M, Shoenfeld Y. Fermented wheat germ extract (Avemar) in the treatment of cancer and autoimmune diseases. Annals of the New York Academy of Sciences. 2005 Jun;1051:529-42.
- Marshall JR. Prevention of colorectal cancer: diet, chemoprevention, and lifestyle. Gastroenterology Clinics of North America. 2008;37(1):73-vi
- Duffield-Lillico AJ, Reid ME et al. Baseline characteristics and the effect of selenium supplementation on cancer incidence in a randomized clinical trial: a summary report of the Nutritional Prevention of Cancer Trial. Cancer Epidemiology, Biomarkers & Prevention. 2002;11(7):630-639.
- Dong J, Liang W et al. Saponins regulate intestinal inflammation in colon cancer and IBD. Pharmacological Research. 2019;144:66–72.
- Reddivari L, Charepalli V et al. Grape compounds suppress colon cancer stem cells in vitro and in a rodent model of colon carcinogenesis. BMC Complementary and Alternative Medicine. 2016 Aug 9;16:278.
- Zick SM, Turgeon DK et al. Phase II study of the effects of ginger root extract on eicosanoids in colon mucosa in people at normal risk for colorectal cancer. Cancer Prevention Research (Philadelphia). 2011 Nov;4(11):1929-37.
- de Lima RMT, Dos Reis AC et al. Protective and therapeutic potential of ginger (Zingiber officinale) extract and [6]-gingerol in cancer: a comprehensive review. Phytotherapy Research. 2018 Oct;32(10):1885-1907; Núñez-Sánchez MA, González-Sarrías A et al. Dietary phenolics against colorectal cancer—from promising preclinical results to poor translation into clinical trials: pitfalls and future needs. Molecular Nutrition and Food Research. 2015 Jul;59(7):1274-91.
- Zhang M, Viennois E et al. Edible ginger-derived nanoparticles: a novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials. 2016 Sep;101:321-40.
- Derry MM, Raina K et al. Grape seed extract efficacy against azoxymethane-induced colon tumorigenesis in A/J mice: interlinking miRNA with cytokine signaling and inflammation. Cancer Prevention Research (Philadelphia). 2013 Jul;6(7):625-33; Derry MM, Raina K, Agarwal C, Agarwal R. Identifying molecular targets of lifestyle modifications in colon cancer prevention. Frontiers in Oncology. 2013 May 14;3:119; Velmurugan B, Singh RP, Agarwal R, Agarwal C. Dietary-feeding of grape seed extract prevents azoxymethane-induced colonic aberrant crypt foci formation in fischer 344 rats. Mol Carcinog. 2010 Jul;49(7):641-52; Velmurugan B, Singh RP, Kaul N, Agarwal R, Agarwal C. Dietary feeding of grape seed extract prevents intestinal tumorigenesis in APCmin/+ mice. Neoplasia. 2010 Jan;12(1):95-102.
- Velmurugan B, Singh RP, Agarwal R, Agarwal C. Dietary-feeding of grape seed extract prevents azoxymethane-induced colonic aberrant crypt foci formation in fischer 344 rats. Molecular Carcinogenesis. 2010 Jul;49(7):641-52.
- Velmurugan B, Singh RP, Kaul N, Agarwal R, Agarwal C. Dietary feeding of grape seed extract prevents intestinal tumorigenesis in APCmin/+ mice. Neoplasia. 2010 Jan;12(1):95-102.
- Satia JA, Littman A, Slatore CG, Galanko JA, White E.Associations of herbal and specialty supplements with lung and colorectal cancer risk in the VITamins and Lifestyle study. Cancer Epidemiology, Biomarkers & Prevention. 2009 May;18(5):1419-28; Zhu B, Zou L, Qi L, Zhong R, Miao X. Allium vegetables and garlic supplements do not reduce risk of colorectal cancer, based on meta-analysis of prospective studies. Clinical Gastroenterology and Hepatology. 2014 Dec;12(12):1991-2001.e1-4; quiz e121.
- Harvie M. Nutritional supplements and cancer: potential benefits and proven harms. American Society of Clinical Oncology Educational Book. 2014;e478-e486.
- Katona BW, Weiss JM. Chemoprevention of colorectal cancer. Gastroenterology. 2020;158(2):368–388; Dolejs SC, Gayed B, Fajardo A. Prevention of colorectal neoplasia. Clinics in Colon and Rectal Surgery. 2016;29(4):353-362.
- Bibbins-Domingo K; US Preventive Services Task Force. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: US Preventive Services Task Force recommendation statement. Annals of Internal Medicine. 2016 Jun 21;164(12):836-45.
- Bosetti C, Santucci C, Gallus S, Martinetti M, La Vecchia C. Aspirin and the risk of colorectal and other digestive tract cancers: an updated meta-analysis through 2019. Annals of Oncology. 2020;S0923-7534(20)36073-7; Bosetti C, Rosato V, Gallus S, Cuzick J, La Vecchia C. Aspirin and cancer risk: a quantitative review to 2011. Annals of Oncology. 2012 Jun;23(6):1403-15; Ye X, Fu J, Yang Y, Chen S. Dose-risk and duration-risk relationships between aspirin and colorectal cancer: a meta-analysis of published cohort studies. PLoS One. 2013;8(2):e57578; Yang T, Li X et al. International Journal of Cancer. Gene-environment interactions and colorectal cancer risk: an umbrella review of systematic reviews and meta-analyses of observational studies. 2019 Nov 1;145(9):2315-2329. doi: 10.1002/ijc.32057; Mohammed A, Yarla NS, Madka V, Rao CV. Clinically relevant anti-inflammatory agents for chemoprevention of colorectal cancer: new perspectives. International Journal of Molecular Sciences. 2018 Aug 8;19(8). pii: E2332; Chen J, Stark LA. Aspirin prevention of colorectal cancer: focus on NF-κB signalling and the nucleolus. Biomedicines. 2017 Jul 18;5(3). pii: E43; Bailie L, Loughrey MB, Coleman HG. Lifestyle risk factors for serrated colorectal polyps: a systematic review and meta-analysis. Gastroenterology. 2017 Jan;152(1):92-104; Piazuelo E, Lanas A. NSAIDS and gastrointestinal cancer. Prostaglandins & Other Lipid Mediators. 2015 Jul;120:91-6; Thanikachalam K, Khan G. Colorectal cancer and nutrition. Nutrients. 2019 Jan 14;11(1). pii: E164; Dolejs SC, Gayed B, Fajardo A. Prevention of colorectal neoplasia. Clinics in Colon and Rectal Surgery. 2016;29(4):353-362; Wakeman C, Keenan J et al. Chemoprevention of colorectal neoplasia. ANZ Journal of Surgery. 2017 Dec;87(12):E228-E232; Pan P, Huang YW et al. Could aspirin and diets high in fiber act synergistically to reduce the risk of colon cancer in humans? International Journal of Molecular Sciences. 2018 Jan 6;19(1). pii: E166; Chapelle N, Martel M, Toes-Zoutendijk E, Barkun AN, Bardou M. Recent advances in clinical practice: colorectal cancer chemoprevention in the average-risk population. Gut. 2020 Sep 28:gutjnl-2020-320990.
- Burn J, Sheth H et al; CAPP2 Investigators. Cancer prevention with aspirin in hereditary colorectal cancer (Lynch syndrome), 10-year follow-up and registry-based 20-year data in the CAPP2 study: a double-blind, randomised, placebo-controlled trial. Lancet. 2020 Jun 13;395(10240):1855-1863.
- Frouws MA, Reimers MS et al. The influence of BRAF and KRAS mutation status on the association between aspirin use and survival after colon cancer diagnosis.PLoS One. 2017 Jan 26;12(1):e0170775; Krstic MN, Mijac DD, Popovic DD, Pavlovic Markovic A, Milosavljević T. General aspects of primary cancer prevention. Digestive Diseases. 2019;37(5):406-415; Baron JA, Cole BF et al. A randomized trial of aspirin to prevent colorectal adenomas. New England Journal of Medicine. 2003 Mar 6;348(10):891-9; Sandler RS, Halabi S et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. New England Journal of Medicine. 2003 Mar 6;348(10):883-90.
- Cooper K, Squires H et al. Chemoprevention of colorectal cancer: systematic review and economic evaluation. Health Technol Assess. 2010 Jun;14(32):1-206; Umezawa S, Higurashi T et al. Chemoprevention of colorectal cancer: past, present, and future. Cancer Science. 2019 Oct;110(10):3018-3026; Serrano D, Bonanni B, Brown K. Therapeutic cancer prevention: achievements and ongoing challenges—a focus on breast and colorectal cancer. Molecular Oncology. 2019 Mar;13(3):579-590; Burn J, Gerdes AM et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet. 2011 Dec 17;378(9809):2081-7.
- Rothwell PM, Cook NR et al. Effects of aspirin on risks of vascular events and cancer according to bodyweight and dose: analysis of individual patient data from randomised trials. Lancet. 2018 Aug 4;392(10145):387-399.
- Loomans-Kropp HA, Pinsky P, Umar A. Evaluation of aspirin use with cancer incidence and survival among older adults in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. JAMA Network Open. 2021 Jan 4;4(1):e2032072.
- Guo CG, Ma W et al. Aspirin use and risk of colorectal cancer among older adults. JAMA Oncol. 2021 Jan 21.
- Mitrugno A, Sylman JL et al. Aspirin therapy reduces the ability of platelets to promote colon and pancreatic cancer cell proliferation: implications for the oncoprotein c-MYC. American Journal of Physiology. Cell Physiology. 2017 Feb 1;312(2):C176-C189; Patrignani P, Patrono C. Aspirin, platelet inhibition and cancer prevention. Platelets. 2018 Dec;29(8):779-785.
- Mitrugno A, Sylman JL et al. Aspirin therapy reduces the ability of platelets to promote colon and pancreatic cancer cell proliferation: implications for the oncoprotein c-MYC. American Journal of Physiology—Cell Physiology. 2017;312(2):C176–C189.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Chang R. Beyond the Magic Bullet: The Anti-Cancer Cocktail. New York: Square One Publishers. 2012.
- Vogtmann E, Corley DA et al. Oral bisphosphonates and colorectal cancer. Scientific Reports. 2017;7:44177; Ibáñez-Sanz G, Guinó E et al. Risk of colorectal cancer in users of bisphosphonates: analysis of population-based electronic health records. European Journal of Epidemiology. 2019;10.1007/s10654-019-00584-5; Eiken P, Vestergaard P. Oral bisphosphonates and colon cancer: an update. Therapeutic Advances in Musculoskeletal Disease. 2015;7(4):160–168; Ma J, Gao S, Ni X, et al. Exposure to bisphosphonates and risk of colorectal cancer. British Journal of Clinical Pharmacology. 2013;76(3):320–328; Thosani N, Thosani SN et al. Reduced risk of colorectal cancer with use of oral bisphosphonates: a systematic review and meta-analysis. Journal of Clinical Oncology. 2013;31(5):623–630; Singh S, Singh AG, Murad MH, Limburg PJ. Bisphosphonates are associated with reduced risk of colorectal cancer: a systematic review and meta-analysis. Clinical Gastroenterology and Hepatology. 2013;11(3):232–9.e1; Oh YH, Yoon C, Park SM. Bisphosphonate use and gastrointestinal tract cancer risk: meta-analysis of observational studies. World Journal of Gastroenterology. 2012;18(40):5779–5788; Dolejs SC, Gayed B, Fajardo A. Prevention of colorectal neoplasia. Clinics in Colon and Rectal Surgery. 2016;29(4):353-362.
- Song M, Chan AT. Environmental factors, gut microbiota, and colorectal cancer prevention. Clinical Gastroenterology and Hepatology. 2019 Jan;17(2):275-289; Katona BW, Weiss JM. Chemoprevention of colorectal cancer. Gastroenterology. 2019 Sep 26. pii: S0016-5085(19)41364-4.
- Joo MK, Park JJ, Chun HJ. Additional benefits of routine drugs on gastrointestinal cancer: statins, metformin, and proton pump inhibitors. Digestive Diseases. 2018;36(1):1-14; Rokkas T, Portincasa P. Colon neoplasia in patients with type 2 diabetes on metformin: a meta-analysis. European Journal of Internal Medicine. 2016 Sep;33:60-6; Krstic MN, Mijac DD, Popovic DD, Pavlovic Markovic A, Milosavljević T. General aspects of primary cancer prevention. Digestive Diseases. 2019;37(5):406-415; Katona BW, Weiss JM. Chemoprevention of colorectal cancer. Gastroenterology. 2019 Sep 26. pii: S0016-5085(19)41364-4; Krstic MN, Mijac DD, Popovic DD, Pavlovic Markovic A, Milosavljević T. General aspects of primary cancer prevention. Digestive Diseases. 2019;37(5):406–415.
- Paleari L, Burhenne J et al. High accumulation of metformin in colonic tissue of subjects with diabetes or the metabolic syndrome. Gastroenterology. 2018 Apr;154(5):1543-1545; Umezawa S, Higurashi T et al. Chemoprevention of colorectal cancer: past, present, and future. Cancer Science. 2019 Oct;110(10):3018-3026; Higurashi T, Hosono K et al. Metformin for chemoprevention of metachronous colorectal adenoma or polyps in post-polypectomy patients without diabetes: a multicentre double-blind, placebo-controlled, randomised phase 3 trial. Lancet Oncology. 2016 Apr;17(4):475-483.
- Higurashi T, Nakajima A. Metformin and colorectal cancer. Frontiers in Endocrinology (Lausanne). 2018 Oct 23;9:622.
- La Vecchia C, Bosetti C. Metformin: are potential benefits on cancer risk extended to cancer survival? The Oncologist. 2013 Dec;18(12):1245-1247.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Piazuelo E, Lanas A. NSAIDs and gastrointestinal cancer. Prostaglandins & Other Lipid Mediators. 2015;120:91–96; Chapelle N, Martel M, Toes-Zoutendijk E, Barkun AN, Bardou M. Recent advances in clinical practice: colorectal cancer chemoprevention in the average-risk population. Gut. 2020 Sep 28:gutjnl-2020-320990.
- Tomić T, Domínguez-López S, Barrios-Rodríguez R. Non-aspirin non-steroidal anti-inflammatory drugs in prevention of colorectal cancer in people aged 40 or older: a systematic review and meta-analysis. Cancer Epidemiology. 2019;58:52–62.
- Wakeman C, Keenan J et al. Chemoprevention of colorectal neoplasia. ANZ Journal of Surgery. 2017 Dec;87(12):E228-E232; Arber N, Eagle CJ et al. Celecoxib for the prevention of colorectal adenomatous polyps. New England Journal of Medicine. 2006 Aug 31;355(9):885-95; Bertagnolli MM, Eagle CJ et al. Celecoxib for the prevention of sporadic colorectal adenomas. New England Journal of Medicine. 2006 Aug 31;355(9):873-84; Mohammed A, Yarla NS, Madka V, Rao CV. Clinically relevant anti-inflammatory agents for chemoprevention of colorectal cancer: new perspectives. International Journal of Molecular Sciences. 2018 Aug 8;19(8). pii: E2332; Dolejs SC, Gayed B, Fajardo A. Prevention of colorectal neoplasia. Clinics in Colon and Rectal Surgery. 2016;29(4):353-362.
- Tomić T, Domínguez-López S, Barrios-Rodríguez R. Non-aspirin non-steroidal anti-inflammatory drugs in prevention of colorectal cancer in people aged 40 or older: a systematic review and meta-analysis. Cancer Epidemiology. 2019;58:52–62.
- Steinbach G, Lynch PM et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. New England Journal of Medicine. 2000 Jun 29;342(26):1946-52.
- Giardiello FM, Hamilton SR et al. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. New England Journal of Medicine. 1993 May 6;328(18):1313-6.
- Tomić T, Domínguez-López S, Barrios-Rodríguez R. Non-aspirin non-steroidal anti-inflammatory drugs in prevention of colorectal cancer in people aged 40 or older: a systematic review and meta-analysis. Cancer Epidemiology. 2019;58:52–62.
- Dolejs SC, Gayed B, Fajardo A. Prevention of colorectal neoplasia. Clinics in Colon and Rectal Surgery. 2016;29(4):353-362.
- Tomić T, Domínguez-López S, Barrios-Rodríguez R. Non-aspirin non-steroidal anti-inflammatory drugs in prevention of colorectal cancer in people aged 40 or older: a systematic review and meta-analysis. Cancer Epidemiology. 2019;58:52–62.
- Liu Y, Jin PP, Sun XC, Hu TT. Thiazolidinediones and risk of colorectal cancer in patients with diabetes mellitus: a meta-analysis. Saudi Journal of Gastroenterol. 2018 Mar-Apr;24(2):75-81.
- Augustin Y, Krishna S, Kumar D, Pantziarka P. The wisdom of crowds and the repurposing of artesunate as an anticancer drug. Ecancermedicalscience. 2015;9:ed50.
- Katona BW, Weiss JM. Chemoprevention of colorectal cancer. Gastroenterology. 2020;158(2):368–388; Joo MK, Park JJ, Chun HJ. Additional benefits of routine drugs on gastrointestinal cancer: statins, metformin, and proton pump inhibitors. Digestive Diseases. 2018;36(1):1-14; Dobrzycka M, Spychalski P et al. Statins and colorectal cancer—a systematic review. Experimental and Clinical Endocrinology & Diabetes. 2018;10.1055/a-0668-5692; Qu B, Qu H. The influence of statins on risk and patient survival in colorectal cancer. Journal of Clinical Gastroenterology. 2019;53(9):699–701; Krstic MN, Mijac DD, Popovic DD, Pavlovic Markovic A, Milosavljević T. General aspects of primary cancer prevention. Digestive Diseases. 2019;37(5):406–415; Lee JW, You NY et al. Statin use and site-specific risk of colorectal cancer in individuals with hypercholesterolemia from the National Health Insurance Service-National Health Screening Cohort (NHIS-HEALS). Nutrition, Metabolism & Cardiovascular Diseases. 2019;29(7):701–709; Ibáñez-Sanz G, Guinó E et al. Statin use and the risk of colorectal cancer in a population-based electronic health records study. Scientific Reports. 2019;9(1):13560; Lai SW, Kuo YH, Fang CW, Liao KF. Statins therapy and colorectal cancer risk. Nutrition, Metabolism & Cardiovascular Diseases. 2019;29(12):1429–1430; Cheung KS, Chen L et al. Statins reduce the progression of non-advanced adenomas to colorectal cancer: a postcolonoscopy study in 187 897 patients. Gut. 2019;68(11):1979–1985; Bonovas S. Statins: do they have a potential role in cancer prevention and modifying cancer-related outcomes? Drugs. 2014 Oct;74(16):1841-8; Gronich N, Rennert G. Beyond aspirin--cancer prevention with statins, metformin and bisphosphonates. Nature Reviews. Clinical Oncology. 2013 Nov;10(11):625-42; Pisanti S, Picardi P, Ciaglia E, D'Alessandro A, Bifulco M. Novel prospects of statins as therapeutic agents in cancer. Pharmacological Research. 2014 Oct;88:84-98; Broughton T, Sington J, Beales IL. Statin use is associated with a reduced incidence of colorectal cancer: a colonoscopy-controlled case-control study. BMC Gastroenterology. 2012 Apr 24;12:36; Lytras T, Nikolopoulos G, Bonovas S. Statins and the risk of colorectal cancer: an updated systematic review and meta-analysis of 40 studies. World Journal of Gastroenterology. 2014 Feb 21;20(7):1858-70; Lochhead P, Chan AT. Statins and colorectal cancer. Clinical Gastroenterology and Hepatology. 2013 Feb;11(2):109-18; quiz e13-4\; Bowles EJA, Yu O et al. Cardiovascular medication use and risks of colon cancer recurrences and additional cancer events: a cohort study. BMC Cancer. 2019;19(1):270; Dolejs SC, Gayed B, Fajardo A. Prevention of colorectal neoplasia. Clinics in Colon and Rectal Surgery. 2016;29(4):353-362; Wakeman C, Keenan J et al. Chemoprevention of colorectal neoplasia. ANZ Journal of Surgery. 2017 Dec;87(12):E228-E232; Chae YK, Yousaf M et al. Statins as anti-cancer therapy; can we translate preclinical and epidemiologic data into clinical benefit? Discovery Medicine. 2015 Dec;20(112):413-27; Coogan PF, Smith J, Rosenberg L. Statin use and risk of colorectal cancer. Journal of the National Cancer Institute. 2007;99(1):32–40; Singh H, Mahmud SM, Turner D, Xue L, Demers AA, Bernstein CN. Long-term use of statins and risk of colorectal cancer: a population-based study. American Journal of Gastroenterology. 2009 Dec;104(12):3015-23.
- Katona BW, Weiss JM. Chemoprevention of colorectal cancer. Gastroenterology. 2020;158(2):368–388; Qu B, Qu H. The influence of statins on risk and patient survival in colorectal cancer. Journal of Clinical Gastroenterology. 2019;53(9):699–701; Liu Y, Tang W et al. Association between statin use and colorectal cancer risk: a meta-analysis of 42 studies. Cancer Causes Control. 2014 Feb;25(2):237-49; Pisanti S, Picardi P, Ciaglia E, D'Alessandro A, Bifulco M. Novel prospects of statins as therapeutic agents in cancer. Pharmacological Research. 2014 Oct;88:84-98; Broughton T, Sington J, Beales IL. Statin use is associated with a reduced incidence of colorectal cancer: a colonoscopy-controlled case-control study. BMC Gastroenterology. 2012 Apr 24;12:36; Samadder NJ, Mukherjee B et al. Risk of colorectal cancer in self-reported inflammatory bowel disease and modification of risk by statin and NSAID use. Cancer. 2011 Apr 15;117(8):1640-8.
- Qu B, Qu H. The influence of statins on risk and patient survival in colorectal cancer. Journal of Clinical Gastroenterology. 2019;53(9):699–701.
- Katona BW, Weiss JM. Chemoprevention of colorectal cancer. Gastroenterology. 2020;158(2):368–388.
- Chang R. Beyond the Magic Bullet: The Anti-Cancer Cocktail. New York: Square One Publishers. 2012.
- Yoshida K, Hashimoto T, Sakai Y, Hashiramoto A. Involvement of the circadian rhythm and inflammatory cytokines in the pathogenesis of rheumatoid arthritis. Journal of Immunology Research. 2014;2014:282495.
- Rich T, Innominato PF et al. Elevated serum cytokines correlated with altered behavior, serum cortisol rhythm, and dampened 24-hour rest-activity patterns in patients with metastatic colorectal cancer. Clinical Cancer Research. 2005 Mar 1;11(5):1757-64.
- Ishikawa H, Saeki T et al. Aged garlic extract prevents a decline of NK cell number and activity in patients with advanced cancer. Journal of Nutrition. 2006 Mar;136(3 Suppl):816S-820S.
- Rahman K, Billington D. Dietary supplementation with aged garlic extract inhibits ADP-induced platelet aggregation in humans. Journal of Nutrition. 2000 Nov;130(11):2662-5.
- Silagy CA, Neil HA. A meta-analysis of the effect of garlic on blood pressure. Journal of Hypertension. 1994 Apr;12(4):463-8.
- Ota A, Ulrih NP. An overview of herbal products and secondary metabolites used for management of type two diabetes. Frontiers in Pharmacology. 2017 Jul 6;8:436.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Ong SKL, Shanmugam MK et al. Focus on formononetin: anticancer potential and molecular targets. Cancers (Basel). 2019;11(5):611; Dong J, Liang W et al. Saponins regulate intestinal inflammation in colon cancer and IBD. Pharmacological Research. 2019;144:66–72.
- Chen M, May BH, Zhou IW, Xue CC, Zhang AL. FOLFOX 4 combined with herbal medicine for advanced colorectal cancer: a systematic review. Phytotherapy Research. 2014;28(7):976–991; Chen M, May BH et al. Oxaliplatin-based chemotherapy combined with traditional medicines for neutropenia in colorectal cancer: a meta-analysis of the contributions of specific plants. Critical Reviews in Oncology/Hematology. 2016;105:18–34; Dong J, Liang W et al. Saponins regulate intestinal inflammation in colon cancer and IBD. Pharmacological Research. 2019;144:66–72.
- Chen MH, May BH, Zhou IW, Zhang AL, Xue CC. Integrative medicine for relief of nausea and vomiting in the treatment of colorectal cancer using oxaliplatin-based chemotherapy: a systematic review and meta-analysis. Phytotherapy Research. 2016;30(5):741–753; Dong J, Liang W et al. Saponins regulate intestinal inflammation in colon cancer and IBD. Pharmacological Research. 2019;144:66–72.
- Wang QZ, Chen XP, Huang JP, Jiang XW. [Effects of couplet medicines (Astragalus membranaceus and jiaozhen) on intestinal barrier in postoperative colorectal cancer patients] [Article in Chinese]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2015;35(11):1307–1312.
- Yoshikawa K, Shimada M et al. The effects of the Kampo medicine (Japanese herbal medicine) "Daikenchuto" on the surgical inflammatory response following laparoscopic colorectal resection. Surgery Today. 2012 Jul;42(7):646-51.
- Chen D, Yang Y, Yang P. Quxie Capsule inhibits colon tumor growth partially through foxo1-mediated apoptosis and immune modulation. Integrative Cancer Therapies. 2019;18:1534735419846377; Yang YF, Xu Y, Wu Y. [Clinical randomized double-blinded controlled study on Quxie Capsule in reducing post-operational relapse and metastasis of colorectal cancer] [Article in Chinese]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2007;27(10):879–882.
- Lagoa R, Silva J, Rodrigues JR, Bishayee A. Advances in phytochemical delivery systems for improved anticancer activity. Biotechnology Advances. 2019 Apr 9. pii: S0734-9750(19)30063-1; Amalraj A, Pius A, Gopi S, Gopi S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—a review. Journal of Traditional and Complementary Medicine. 2016 Jun 15;7(2):205-233; Xu B, Yu L, Zhao LZ. Curcumin up regulates T helper 1 cells in patients with colon cancer. American Journal of Translational Research. 2017 Apr 15;9(4):1866-1875; Núñez-Sánchez MA, González-Sarrías A et al. Dietary phenolics against colorectal cancer—from promising preclinical results to poor translation into clinical trials: pitfalls and future needs. Molecular Nutrition & Food Research. 2015 Jul;59(7):1274-91; Hou TY, Davidson LA et al. Nutrient-gene interaction in colon cancer, from the membrane to cellular physiology. Annual Review of Nutrition. 2016 Jul 17;36:543-70; Panahi Y, Saadat A, Beiraghdar F, Sahebkar A. Adjuvant therapy with bioavailability-boosted curcuminoids suppresses systemic inflammation and improves quality of life in patients with solid tumors: a randomized double-blind placebo-controlled trial. Phytotherapy Research. 2014 Oct;28(10):1461-7; Fadus MC, Lau C, Bikhchandani J, Lynch HT. Curcumin: an age-old anti-inflammatory and anti-neoplastic agent. Journal of Traditional and Complementary Medicine. 2016 Sep 9;7(3):339-346; Das J, Ramani R, Suraju MO. Polyphenol compounds and PKC signaling. Biochimica et Biophysica Acta. 2016 Oct;1860(10):2107-21.
- Panahia Y, Saadat A et al. Antioxidant effects of bioavailability-enhanced curcuminoids in patients with solid tumors: a randomized double-blind placebo-controlled trial. Journal of Functional Foods. 2014 Jan;6:615-622.
- Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS Journal. 2013 Jan;15(1):195-218; Carroll RE, Benya RV et al. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prevention Research (Philadelphia, Pennsylvania). 2011 Mar;4(3):354-64.
- He ZY, Shi CB et al. Upregulation of p53 expression in patients with colorectal cancer by administration of curcumin. Cancer Investigation. 2011 Mar;29(3):208-13; Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS Journal . 2013 Jan;15(1):195-218; Carroll RE, Benya RV et al. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prevention Research (Philadelphia, Pennsylvania). 2011 Mar;4(3):354-64. Imran M, Ullah A et al. Cucurmin, anticancer, & antitumor perspectives: a comprehensive review. Critical Reviews in Food Science and Nutrition. 2018 May 24;58(8):1271-1293; Pabla B, Bissonnette M, Konda VJ. Colon cancer and the epidermal growth factor receptor: current treatment paradigms, the importance of diet, and the role of chemoprevention. World Journal of Clinical Oncology. 2015 Oct 10;6(5):133-41; Hou TY, Davidson LA et al. Nutrient-gene interaction in colon cancer, from the membrane to cellular physiology. Annual Review of Nutrition. 2016 Jul 17;36:543-70.
- Lagoa R, Silva J, Rodrigues JR, Bishayee A. Advances in phytochemical delivery systems for improved anticancer activity. Biotechnology Advances. 2019 Apr 9. pii: S0734-9750(19)30063-1.
- McFadden RM, Larmonier CB et al. The role of curcumin in modulating colonic microbiota during colitis and colon cancer prevention. Inflammatory Bowel Diseases. 2015 Nov;21(11):2483-94.
- Alschuler LN, Gazella KA. The Definitive Guide to Cancer, 3rd Edition: An Integrative Approach to Prevention, Treatment, and Healing. Berkeley, California: Celestial Arts. 2010; Alschuler LN, Gazella KA. The Definitive Guide to Thriving after Cancer: A Five-Step Integrative Plan to Reduce the Risk of Recurrence and Build Lifelong Health. Berkeley, California: Ten Speed Press. 2013.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Mueller T, Voigt W. Fermented wheat germ extract—nutritional supplement or anticancer drug? Nutrition Journal. 2011 Sep 5;10:89.
- Mueller T, Voigt W. Fermented wheat germ extract—nutritional supplement or anticancer drug? Nutrition Journal. 2011 Sep 5;10:89; Telekes A, Hegedus M, Chae CH, Vékey K. Avemar (wheat germ extract) in cancer prevention and treatment. Nutrition and Cancer. 2009;61(6):891-9.
- Núñez-Sánchez MA, González-Sarrías A et al. Dietary phenolics against colorectal cancer—from promising preclinical results to poor translation into clinical trials: pitfalls and future needs. Molecular Nutrition and Food Research. 2015 Jul;59(7):1274-91; Alsherbiny MA, Abd-Elsalam WH et al. Ameliorative and protective effects of ginger and its main constituents against natural, chemical and radiation-induced toxicities: a comprehensive review. Food and Chemical Toxicology. 2019 Jan;123:72-97; López-Romero D, Izquierdo-Vega JA. Evidence of some natural products with antigenotoxic effects. part 2: plants, vegetables, and natural resin. Nutrients. 2018 Dec 10;10(12). pii: E1954; Mahomoodally MF, Aumeeruddy MZ et al. Ginger and its active compounds in cancer therapy: from folk uses to nano-therapeutic applications. Seminars in Cancer Biology. 2019 Aug 11. pii: S1044-579X(19)30213-5.
- de Lima RMT, Dos Reis AC et al. Protective and therapeutic potential of ginger (Zingiber officinale) extract and [6]-gingerol in cancer: a comprehensive review. Phytotherapy Research. 2018 Oct;32(10):1885-1907; Núñez-Sánchez MA, González-Sarrías A et al. Dietary phenolics against colorectal cancer—from promising preclinical results to poor translation into clinical trials: pitfalls and future needs. Molecular Nutrition and Food Research. 2015 Jul;59(7):1274-91; Alsherbiny MA, Abd-Elsalam WH et al. Ameliorative and protective effects of ginger and its main constituents against natural, chemical and radiation-induced toxicities: a comprehensive review. Food and Chemical Toxicology. 2019 Jan;123:72-97; Kim Y, Kim DM, Kim JY. Ginger extract suppresses inflammatory response and maintains barrier function in human colonic epithelial caco-2 cells exposed to inflammatory mediators. Journal of Food Science. 2017 May;82(5):1264-1270; Mahomoodally MF, Aumeeruddy MZ et al. Ginger and its active compounds in cancer therapy: from folk uses to nano-therapeutic applications. Seminars in Cancer Biology. 2019 Aug 11. pii: S1044-579X(19)30213-5
- Zhang M, Viennois E et al. Edible ginger-derived nanoparticles: a novel therape utic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials. 2016 Sep;101:321-40.
- Kim Y, Kim DM, Kim JY. Ginger extract suppresses inflammatory response and maintains barrier function in human colonic epithelial caco-2 cells exposed to inflammatory mediators. Journal of Food Science. 2017 May;82(5):1264-1270.
- Mahomoodally MF, Aumeeruddy MZ et al. Ginger and its active compounds in cancer therapy: from folk uses to nano-therapeutic applications. Seminars in Cancer Biology. 2019 Aug 11. pii: S1044-579X(19)30213-5.
- Almatroudi A, Alsahli MA, Alrumaihi F, Allemailem KS, Rahmani AH. Ginger: a novel strategy to battle cancer through modulating cell signalling pathways: a review. Current Pharmaceutical Biotechnology. 2019;20(1):5-16.
- Wee LH, Morad NA et al. Mechanism of chemoprevention against colon cancer cells using combined gelam honey and ginger extract via mTOR and Wnt/β-catenin pathways. Asian Pacific Journal of Cancer Prevention. 2015;16(15):6549-56.
- Alschuler LN, Gazella KA. The Definitive Guide to Cancer, 3rd Edition: An Integrative Approach to Prevention, Treatment, and Healing. Berkeley, California: Celestial Arts. 2010; Alschuler LN, Gazella KA. The Definitive Guide to Thriving after Cancer: A Five-Step Integrative Plan to Reduce the Risk of Recurrence and Build Lifelong Health. Berkeley, California: Ten Speed Press. 2013.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- McKinney N. Naturopathic Oncology, 3rd Edition. Victoria, BC, Canada: Liaison Press. 2016.
- de Urbina JJO, San-Miguel B et al. Effects of oral glutamine on inflammatory and autophagy responses in cancer patients treated with abdominal radiotherapy: a pilot randomized trial. International Journal of Medical Sciences. 2017 Sep 4;14(11):1065-1071.
- Rotovnik Kozjek N, Kompan L, Žagar T, Mrevlje Ž. Influence of enteral glutamine on inflammatory and hormonal response in patients with rectal cancer during preoperative radiochemotherapy. European Journal of Clinical Nutrition. 2017;71(5):671-673.
- Jolfaie NR, Mirzaie S, Ghiasvand R, Askari G, Miraghajani M. The effect of glutamine intake on complications of colorectal and colon cancer treatment: a systematic review. Journal of Research in Medical Sciences. 2015 Sep;20(9):910-8.
- van Stijn MFM, Soeters MR et al. Effects of a carbohydrate-, glutamine-, and antioxidant-enriched oral nutrition supplement on major surgery-induced insulin resistance: a randomized pilot study. JPEN. Journal of Parenteral and Enteral Nutrition. 2018 May;42(4):719-729.
- Cui Y, Hu L et al. Intravenous alanyl-L-glutamine balances glucose-insulin homeostasis and facilitates recovery in patients undergoing colonic resection: a randomised controlled trial. European Journal of Anaesthesiology. 2014 Apr;31(4):212-8.
- Szpetnar M, Matras P et al. Is additional enrichment of diet in branched-chain amino acids or glutamine beneficial for patients receiving total parenteral nutrition after gastrointestinal cancer surgery? Advances in Clinical and Experimental Medicine. 2014 May-Jun;23(3):423-31.
- Jin JW, Inoue O et al. Grape seed extracts inhibit platelet aggregation by inhibiting protein tyrosine phosphatase. Clinical and Applied Thrombosis/Hemostasis. Apr 2014;20(3):278-284; Bijak M, Bobrowski M et al. Anticoagulant effect of polyphenols-rich extracts from black chokeberry and grape seeds. Fitoterapia. 2011 Sep;82(6):811-7; de Lange DW, Scholman WL, Kraaijenhagen RJ, Akkerman JW, van de Wiel A. Alcohol and polyphenolic grape extract inhibit platelet adhesion in flowing blood. European Journal of Clinical Investigation. 2004 Dec;34(12):818-24; de Lange DW, Verhoef S et al. Polyphenolic grape extract inhibits platelet activation through PECAM-1: an explanation for the French paradox. Alcoholism, Clinical and Experimental Research. 2007 Aug;31(8):1308-14.
- Derry MM, Raina K, Agarwal C, Agarwal R. Identifying molecular targets of lifestyle modifications in colon cancer prevention. Frontiers in Oncology. 2013 May 14;3:119; Katiyar SK, Athar M. Grape seeds: ripe for cancer chemoprevention. Cancer Prevention Research (Philadelphin, PA). 2013 Jul;6(7):617-21.
- Derry MM, Raina K et al. Grape seed extract efficacy against azoxymethane-induced colon tumorigenesis in A/J mice: interlinking miRNA with cytokine signaling and inflammation. Cancer Prevention Research (Philadelphia). 2013 Jul;6(7):625-33; Derry MM, Raina K, Agarwal C, Agarwal R. Identifying molecular targets of lifestyle modifications in colon cancer prevention. Frontiers in Oncology. 2013 May 14;3:119.
- Katiyar SK, Athar M. Grape seeds: ripe for cancer chemoprevention. Cancer Prevention Research (Philadelphin, PA). 2013 Jul;6(7):617-21.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Ni J, Guo X, Wang H, Zhou T, Wang X. Differences in the effects of EGCG on chromosomal stability and cell growth between normal and colon cancer cells. Molecules. 2018 Mar 29;23(4). pii: E788; Carini F, Tomasello G et al. Colorectal cancer and inflammatory bowel diseases: effects of diet and antioxidants. Journal of Biological Regulators and Homeostatic Agents. 2017 Jul-Sep;31(3):791-795; Kuppusamy P, Yusoff MM et al. Nutraceuticals as potential therapeutic agents for colon cancer: a review. Acta Pharm Sin B. 2014 Jun;4(3):173-81; Fajardo AM, Piazza GA. Chemoprevention in gastrointestinal physiology and disease. Anti-inflammatory approaches for colorectal cancer chemoprevention. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2015 Jul 15;309(2):G59-70; Massounga Bora AF, Ma S, Li X, Liu L Application of microencapsulation for the safe delivery of green tea polyphenols in food systems: review and recent advances. Food Research International. 2018 Mar;105:241-249.
- Liu C, Li P et al. Advances in the antagonism of epigallocatechin-3-gallate in the treatment of digestive tract tumors. Molecules. 2019 May 3;24(9). pii: E1726; Fajardo AM, Piazza GA. Chemoprevention in gastrointestinal physiology and disease. Anti-inflammatory approaches for colorectal cancer chemoprevention. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2015 Jul 15;309(2):G59-70; Shirakami Y, Ohnishi M, Sakai H, Tanaka T, Shimizu M. Prevention of colorectal cancer by targeting obesity-related disorders and inflammation. International Journal of Molecular Sciences. 2017 Apr 26;18(5). pii: E908.
- Massounga Bora AF, Ma S, Li X, Liu L Application of microencapsulation for the safe delivery of green tea polyphenols in food systems: review and recent advances. Food Research International. 2018 Mar;105:241-249.
- Ni J, Guo X, Wang H, Zhou T, Wang X. Differences in the effects of EGCG on chromosomal stability and cell growth between normal and colon cancer cells. Molecules. 2018 Mar 29;23(4). pii: E788.
- Fajardo AM, Piazza GA. Chemoprevention in gastrointestinal physiology and disease. Anti-inflammatory approaches for colorectal cancer chemoprevention. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2015 Jul 15;309(2):G59-70.
- Zheng XX, Xu YL et al. Effects of green tea catechins with or without caffeine on glycemic control in adults: a meta-analysis of randomized controlled trials. American Journal of Clinical Nutrition. 2013 Apr;97(4):750-62; Kim Y, Keogh JB, Clifton PM. Polyphenols and glycemic control. Nutrients. 2016 Jan 5;8(1). pii: E17; Liu K, Zhou R et al. Effect of green tea on glucose control and insulin sensitivity: a meta-analysis of 17 randomized controlled trials. American Journal of Clinical Nutrition. 2013 Aug;98(2):340-8.
- Yu J, Song P, Perry R, Penfold C, Cooper AR. The effectiveness of green tea or green tea extract on insulin resistance and glycemic control in type 2 diabetes mellitus: a meta-analysis. Diabetes & Metabolism Journal. 2017 Aug;41(4):251-262.
- Alschuler LN, Gazella KA. The Definitive Guide to Cancer, 3rd Edition: An Integrative Approach to Prevention, Treatment, and Healing. Berkeley, California: Celestial Arts. 2010; Alschuler LN, Gazella KA. The Definitive Guide to Thriving after Cancer: A Five-Step Integrative Plan to Reduce the Risk of Recurrence and Build Lifelong Health. Berkeley, California: Ten Speed Press. 2013.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- McKinney N. Naturopathic Oncology, 3rd Edition. Victoria, BC, Canada: Liaison Press. 2016.
- Yehia R, Saleh S, El Abhar H, Saad AS, Schaalan M. L-Carnosine protects against oxaliplatin-induced peripheral neuropathy in colorectal cancer patients: a perspective on targeting Nrf-2 and NF-κB pathways. Toxicology and Applied Pharmacology. 2019;365:41-50.
- Zhong Z, Wheeler MD et al. L-glycine: a novel antiinflammatory, immunomodulatory, and cytoprotective agent. Current Opinions in Clinical Nutrition and Metabolic Care. 2003 Mar;6(2):229-40.
- Pappalardo G, Almeida A, Ravasco P. Eicosapentaenoic acid in cancer improves body composition and modulates metabolism. Nutrition. 2015 Apr;31(4):549-55; de Aguiar Pastore Silva J, Emilia de Souza Fabre M, Waitzberg DL. Omega-3 supplements for patients in chemotherapy and/or radiotherapy: a systematic review. Clinical Nutrition. 2015 Jun;34(3):359-66.
- Sorensen LS, Thorlacius-Ussing O et al. Effects of perioperative supplementation with omega-3 fatty acids on leukotriene B₄ and leukotriene B₅ production by stimulated neutrophils in patients with colorectal cancer: a randomized, placebo-controlled intervention trial. Nutrients. 2014 Sep 29;6(10):4043-57; Silva Jde A, Trindade EB et al. Fish oil supplement alters markers of inflammatory and nutritional status in colorectal cancer patients. Nutrition and Cancer. 2012;64(2):267-73; Xie H, Chang YN. Omega-3 polyunsaturated fatty acids in the prevention of postoperative complications in colorectal cancer: a meta-analysis. OncoTargets and Therapy. 2016 Dec 9;9:7435-7443; Golkhalkhali B, Rajandram R et al. Strain-specific probiotic (microbial cell preparation) and omega-3 fatty acid in modulating quality of life and inflammatory markers in colorectal cancer patients: a randomized controlled trial. Asia-Pacific Journal of Clinical Oncology. 2018 Jun;14(3):179-191.
- Mocellin MC, Camargo CQ, Nunes EA, et al. A systematic review and meta-analysis of the n-3 polyunsaturated fatty acids effects on inflammatory markers in colorectal cancer. Clinical Nutrition. 2016;35:359–369.
- Liang B, Wang S, Impact of postoperative omega-3 fatty acid-supplemented parenteral nutrition on clinical outcomes and immunomodulations in colorectal cancer patients. World Journal of Gastroenterology. 2008 Apr 21;14(15):2434-9.
- Aisu N, Tanimura S et al. Impact of perioperative probiotic treatment for surgical site infections in patients with colorectal cancer. Experimental and Therapeutic Medicine. 2015;10(3):966–972; Roller M, Clune Y, Collins K, Rechkemmer G, Watzl B. Consumption of prebiotic inulin enriched with oligofructose in combination with the probiotics Lactobacillus rhamnosus and Bifidobacterium lactis has minor effects on selected immune parameters in polypectomised and colon cancer patients. British Journal of Nutrition. 2007;97(4):676–684; Anderson SW, Bazzell AF, Dains JE. An integrative review on the effect of prebiotics, probiotics, and synbiotics on infection after colorectal cancer surgery. AORN Journal. 2018;107(2):237–248.
- Aisu N, Tanimura S et al. Impact of perioperative probiotic treatment for surgical site infections in patients with colorectal cancer. Experimental and Therapeutic Medicine. 2015;10(3):966–972.
- Nio Y, Tsubono M et al. Immunomodulation by orally administered protein-bound polysaccharide Krestin (PSK)™ in patients with gastrointestinal cancer. Biotherapy. 1992;4:117–128.
- Nio Y, Tsubono M et al. Immunomodulation by orally administered protein-bound polysaccharide PSK in patients with gastrointestinal cancer. Biotherapy. 1992;4(2):117-28; Evidence-Based Monographs: Professional Resource: Coriolus Versicolor. Ottawa Integrative Cancer Centre. Viewed June 6, 2019.
- Mayland CR, Bennett MI, Allan K. Vitamin C deficiency in cancer patients. Palliative Medicine.2005;19:17–20. with improvements seen after intravenous vitamin C treatmentMikirova N, Casciari J, Riordan N, Hunninghake R. Clinical experience with intravenous administration of ascorbic acid: achievable levels in blood for different states of inflammation and disease in cancer patients. Journal of Translational Medicine. 2013;11:191.
- Hanson MG, Ozenci V et al. A short-term dietary supplementation with high doses of vitamin E increases NK cell cytolytic activity in advanced colorectal cancer patients. Cancer Immunology, Immunotherapy. 2007;56(7):973-984; Malmberg KJ, Lenkei R et al. A short-term dietary supplementation of high doses of vitamin E increases T helper 1 cytokine production in patients with advanced colorectal cancer. Clinical Cancer Research. 2002 Jun;8(6):1772-8.
- Lasry A, Zinger A, Ben-Neriah Y. Inflammatory networks underlying colorectal cancer. Nature Immunology. 2016 Mar;17(3):230-40; Grancher A, Michel P, Di Fiore F, Sefrioui D. [Aspirin and colorectal cancer]. Article in French. Bulletin du Cancer. 2018 Feb;105(2):171-180; Pan P, Huang YW et al. Could aspirin and diets high in fiber act synergistically to reduce the risk of colon cancer in humans? International Journal of Molecular Sciences. 2018 Jan 6;19(1). pii: E166; Chen J, Stark LA. Aspirin prevention of colorectal cancer: focus on NF-κB signalling and the nucleolus. Biomedicines. 2017 Jul 18;5(3). pii: E43.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Bader JE, Enos RT, Velázquez KT, et al. Macrophage depletion using clodronate liposomes decreases tumorigenesis and alters gut microbiota in the AOM/DSS mouse model of colon cancer. American Journal of Physiology—Gastrointestinal and Liver Physiology. 2018;314(1):G22–G31.
- Bader JE, Enos RT, Velázquez KT, et al. Macrophage depletion using clodronate liposomes decreases tumorigenesis and alters gut microbiota in the AOM/DSS mouse model of colon cancer. American Journal of Physiology—Gastrointestinal and Liver Physiology. 2018;314(1):G22–G31.
- Losurdo G, Principi M et al. Histamine and histaminergic receptors in colorectal cancer: from basic science to evidence-based medicine. Anti-Cancer Agents in Medicinal Chemistry. 2018;18(1):15–20; Pantziarka P, Bouche G, Meheus L, Sukhatme V, Sukhatme VP. Repurposing drugs in oncology (ReDO)—cimetidine as an anti-cancer agent. Ecancermedicalscience. 2014;8:485; Eaton D, Hawkins RE. Cimetidine in colorectal cancer--are the effects immunological or adhesion-mediated? British Journal of Cancer. 2002;86(2):159–160.
- Lin CY, Bai DJ, Yuan HY, et al. Perioperative cimetidine administration promotes peripheral blood lymphocytes and tumor infiltrating lymphocytes in patients with gastrointestinal cancer: results of a randomized controlled clinical trial. World Journal of Gastroenterology. 2004;10(1):136–142; Li B, Cao F, Zhu Q, et al. Perioperative cimetidine administration improves systematic immune response and tumor infiltrating lymphocytes in patients with colorectal cancer. Hepatogastroenterology 2013;60:244–247; Kumar A. Cimetidine: an immunomodulator. DICP. 1990 Mar;24(3):289-95.
- Eaton D, Hawkins RE. Cimetidine in colorectal cancer--are the effects immunological or adhesion-mediated? British Journal of Cancer. 2002;86(2):159–160.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Lemole G, Mehta P, McKee D. After Cancer Care: The Definitive Self-Care Guide to Getting and Staying Well for Patients with Cancer. New York, New York: Rodale, Inc. 2015.
- Brewer GJ. Anticopper therapy against cancer and diseases of inflammation and fibrosis. Drug Discovery Today. 2005;10(16):1103–1109.
- Khan G, Merajver S. Copper chelation in cancer therapy using tetrathiomolybdate: an evolving paradigm. Expert Opinion on Investigational Drugs. 2009;18(4):541–548.
- Joo MK, Park JJ, Chun HJ. Additional benefits of routine drugs on gastrointestinal cancer: statins, metformin, and proton pump inhibitors. Digestive Diseases. 2018;36(1):1-14.
- Umezawa S, Higurashi T et al. Chemoprevention of colorectal cancer: past, present, and future. Cancer Science. 2019 Oct;110(10):3018-3026.
- Cui G, Zhang T et al. High blood glucose levels correlate with tumor malignancy in colorectal cancer patients. Medical Science Monitor. 2015 Dec 8;21:3825-33; Perrigue MM, Drewnowski A et al. Randomized trial testing the effects of eating frequency on two hormonal biomarkers of metabolism and energy balance. Nutrition and Cancer. 2017 Jan;69(1):56-63.
- Wolpin BM, Meyerhardt JA et al. Insulin, the insulin-like growth factor axis, and mortality in patients with nonmetastatic colorectal cancer. Journal of Clinical Oncology. 2009 Jan 10;27(2):176-85; Sax AT, Jenkins DG et al. The insulin-like growth factor axis: a biological mechanism linking physical activity to colorectal cancer survival. Cancer Epidemiology. 2014 Aug;38(4):455-9; Yuan C, Bao Y et al. Influence of dietary insulin scores on survival in colorectal cancer patients. British Journal of Cancer. 2017 Sep 26;117(7):1079-1087.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- McKinney N. Naturopathic Oncology, 3rd Edition. Victoria, BC, Canada: Liaison Press. 2016.
- Lasry A, Zinger A, Ben-Neriah Y. Inflammatory networks underlying colorectal cancer. Nature Immunology. 2016;17(3):230–240.
- Tomić T, Domínguez-López S, Barrios-Rodríguez R. Non-aspirin non-steroidal anti-inflammatory drugs in prevention of colorectal cancer in people aged 40 or older: a systematic review and meta-analysis. Cancer Epidemiology. 2019;58:52–62.
- Buijsen J, van den Bogaard J et al. A phase I-II study on the combination of rapamycin and short course radiotherapy in rectal cancer. Radiotherapy & Oncology. 2015;116(2):214–220.
- Pisanti S, Picardi P, Ciaglia E, D'Alessandro A, Bifulco M. Novel prospects of statins as therapeutic agents in cancer. Pharmacological Research. 2014 Oct;88:84-98.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Treatment. New York: Bantam Dell. 2009.
- Pei X, Zhou Z, Xu G. [Effect of electroacupuncture for immune function of patients treated with laparoscopic radical rectectomy for rectal cancer] [Article in Chinese]. Zhongguo Zhen Jiu. 2016;36(6):613-616.
- Csiszar A, Labinskyy N, Jimenez R, et al. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: role of circulating factors and SIRT1. Mechanisms of Ageing and Development. 2009;130(8):518–527.
- Nencioni A, Caffa I, Cortellino S, Longo VD. Fasting and cancer: molecular mechanisms and clinical application. Nature Reviews. Cancer. 2018;18(11):707–719; Mansell PI, Macdonald IA. The effect of starvation on insulin-induced glucose disposal and thermogenesis in humans. Metabolism. 1990;39(5):502–510; Romijn JA, Godfried MH, Hommes MJ, Endert E, Sauerwein HP. Decreased glucose oxidation during short-term starvation. Metabolism. 1990;39(5):525–530; Ngo TH, Barnard RJ, Tymchuk CN, Cohen P, Aronson WJ. Effect of diet and exercise on serum insulin, IGF-I, and IGFBP-1 levels and growth of LNCaP cells in vitro (United States). Cancer Causes & Control. 2002;13(10):929–935.
- Ngo TH, Barnard RJ, Tymchuk CN, Cohen P, Aronson WJ. Effect of diet and exercise on serum insulin, IGF-I, and IGFBP-1 levels and growth of LNCaP cells in vitro (United States). Cancer Causes & Control. 2002;13(10):929–935; Sun P, Wang H, He Z, et al. Fasting inhibits colorectal cancer growth by reducing M2 polarization of tumor-associated macrophages. Oncotarget. 2017;8(43):74649–74660.
- Buono R, Longo VD. Starvation, stress resistance, and cancer. Trends in Endocrinology and Metabolism. 2018;29(4):271–280; Sun P, Wang H et al. Fasting inhibits colorectal cancer growth by reducing M2 polarization of tumor-associated macrophages. Oncotarget. 2017;8(43):74649–74660.
- Lu SSM, Mohammed Z et al. Antibiotics use and subsequent risk of colorectal cancer: a Swedish nationwide population-based study. JNCI: Journal of the National Cancer Institute. 2021 Sep 1:djab125.
- Zhang J, Haines C et al. Oral antibiotic use and risk of colorectal cancer in the United Kingdom, 1989-2012: a matched case-control study. Gut. 2019;68(11):1971–1978.
- Harrison P. Antibiotics use and increased risk of colon cancer? Medscape. August 23, 2019. Viewed February 25, 2020.
- Song M, Chan AT. Environmental factors, gut microbiota, and colorectal cancer prevention. Clinical Gastroenterology and Hepatology. 2019;17(2):275–289.
- Block KI, Block PB, Gyllenhaal C. Integrative treatment for colorectal cancer: a comprehensive approach. Journal of Alternative and Complementary Medicine. 2018;24(9-10):890–901.
- Block KI, Block PB, Gyllenhaal C. Integrative treatment for colorectal cancer: a comprehensive approach. Journal of Alternative and Complementary Medicine. 2018;24(9-10):890–901.
- Purcell RV, Pearson J et al. Colonization with enterotoxigenic Bacteroides fragilis is associated with early-stage colorectal neoplasia. PLoS One. 2017;12(2):e0171602.
- Block KI, Block PB, Gyllenhaal C. Integrative treatment for colorectal cancer: a comprehensive approach. Journal of Alternative and Complementary Medicine. 2018;24(9-10):890–901; Bultman SJ. Emerging roles of the microbiome in cancer. Carcinogenesis. 2014;35(2):249–255.
- Chattopadhyay I, Dhar R et al. Exploring the role of gut microbiome in colon cancer. Applied Biochemistry and Biotechnology. 2021 Jan 25; Dejea C, Wick E, Sears CL. Bacterial oncogenesis in the colon. Future Microbiology. 2013;8(4):445–460.
- Purcell RV, Pearson J et al. Colonization with enterotoxigenic Bacteroides fragilis is associated with early-stage colorectal neoplasia. PLoS One. 2017;12(2):e0171602.
- Melnyk M, Casey RG, Black P, Koupparis AJ. Enhanced recovery after surgery (ERAS) protocols: time to change practice? Canadian Urological Association Journal. 2011;5(5):342-348.
- Tebala GD, Gordon-Dixon A, Imtiaz M, Shrestha A, Toeima M. Enhanced recovery after rectal surgery: what we have learned so far. Mini-invasive Surgery. 2018;2:32.
- Tebala GD, Gordon-Dixon A, Imtiaz M, Shrestha A, Toeima M. Enhanced recovery after rectal surgery: what we have learned so far. Mini-invasive Surgery. 2018;2:32.
- Tebala GD, Gordon-Dixon A, Imtiaz M, Shrestha A, Toeima M. Enhanced recovery after rectal surgery: what we have learned so far. Mini-invasive Surgery. 2018;2:32; Calder PC. Immunonutrition. BMJ. 2003;327(7407):117-118.
- López Hellín J, Baena-Fustegueras JA, Sabín-Urkía P, Schwartz-Riera S, García-Arumí E. Nutritional modulation of protein metabolism after gastrointestinal surgery. European Journal of Clinical Nutrition. 2008;62(2):254-262.
- Gustafsson UO, Scott MJ et al. Guidelines for perioperative care in elective colorectal surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations: 2018. World Journal of Surgery. 2019;43(3):659-695.
- Ogbonna CI, Lembke A. Tapering patients off of benzodiazepines. American Family Physician. 2017 Nov 1;96(9):606-610.
- Carmichael JC, Keller DS et al. Clinical practice guidelines for enhanced recovery after colon and rectal surgery from the American Society of Colon and Rectal Surgeons and Society of American Gastrointestinal and Endoscopic Surgeons. Diseases of the Colon & Rectum. 2017;60(8):761-784.
- Minnella EM, Carli F. Prehabilitation and functional recovery for colorectal cancer patients. European Journal of Surgical Oncology. 2018;44(7):919-926.
- Minnella EM, Carli F. Prehabilitation and functional recovery for colorectal cancer patients. European Journal of Surgical Oncology. 2018;44(7):919-926.
- Kim SY, Kim NK et al. Effects of postoperative pain management on immune function after laparoscopic resection of colorectal cancer: a randomized study. Medicine (Baltimore). 2016 Jun 17;95(24):e4641.
- Haldar R, Ben-Eliyahu S. Reducing the risk of post-surgical cancer recurrence: a perioperative anti-inflammatory anti-stress approach. Future Oncology. 2018;14(11):1017-1021; Khanna AK, Perez ER, Laudanski K, Moraska A, Cummings KC. Perioperative care and cancer recurrence: Is there a connection? World Journal of Anesthesiology. 2014;3(1):31-45; Hiller JG, Perry NJ, Poulogiannis G, Riedel B, Sloan EK. Perioperative events influence cancer recurrence risk after surgery. Nature Reviews Clinical Oncology. 2018 Apr;15(4):205-218; Dang Y, Shi X, Xu W, Zuo M. The effect of anesthesia on the immune system in colorectal cancer patients. Canadian Journal of Gastroenterology & Hepatology. 2018 Apr 1;2018:7940603.
- Hiller JG, Perry NJ, Poulogiannis G, Riedel B, Sloan EK. Perioperative events influence cancer recurrence risk after surgery. Nature Reviews. Clinical Oncology. 2018;15(4):205-218.
- Sikorszki L, Bezsilla J, Vajda K, Temesi R. Lokálisan előrehaladott kemoirradiált rectumtumorok nyitott és laparoszkópos műtéteinek az összehasonlítása [The comparison of open and laparoscopic surgeries of the locally advanced chemo-irradiated rectum tumours]. Magyar Sebészet. 2020;73(1):29-36.
- Khanna AK, Perez ER, Laudanski K, Moraska A, Cummings KC. Perioperative care and cancer recurrence: Is there a connection? World Journal of Anesthesiology. 2014;3(1):31-45; Dang Y, Shi X, Xu W, Zuo M. The effect of anesthesia on the immune system in colorectal cancer patients. Canadian Journal of Gastroenterology & Hepatology. 2018 Apr1;2018:7940603.
- Dang Y, Shi X, Xu W, Zuo M. The effect of anesthesia on the immune system in colorectal cancer patients. Canadian Journal of Gastroenterology & Hepatology. 2018 Apr 1;2018:7940603; Gelman D, Gelmanas A et al. Role of multimodal analgesia in the evolving enhanced recovery after surgery pathways. Medicina. 2018 May; 54(2): 20.
- Khanna AK, Perez ER, Laudanski K, Moraska A, Cummings KC. Perioperative care and cancer recurrence: Is there a connection? World Journal of Anesthesiology. 2014;3(1):31-45.
- Khanna AK, Perez ER, Laudanski K, Moraska A, Cummings KC. Perioperative care and cancer recurrence: Is there a connection? World Journal of Anesthesiology. 2014;3(1):31-45; Hiller JG, Perry NJ, Poulogiannis G, Riedel B, Sloan EK. Perioperative events influence cancer recurrence risk after surgery. Nature Reviews Clinical Oncology. 2018 Apr;15(4):205-218; Dang Y, Shi X, Xu W, Zuo M. The Effect of Anesthesia on the Immune System in Colorectal Cancer Patients. Canadian Journal of Gastroenterology & Hepatology. 2018 Apr 1;2018:7940603.
- Khanna AK, Perez ER, Laudanski K, Moraska A, Cummings KC. Perioperative care and cancer recurrence: Is there a connection? World Journal of Anesthesiology. 2014;3(1):31-45; Hiller JG, Perry NJ, Poulogiannis G, Riedel B, Sloan EK. Perioperative events influence cancer recurrence risk after surgery. Nature Reviews Clinical Oncology. 2018 Apr;15(4):205-218; Dang Y, Shi X, Xu W, Zuo M. The effect of anesthesia on the immune system in colorectal cancer patients. Canadian Journal of Gastroenterology & Hepatology. 2018 Apr 1;2018:7940603.
- Richards CH, Platt JJ et al. The impact of perioperative risk, tumor pathology and surgical complications on disease recurrence following potentially curative resection of colorectal cancer. Annals of Surgery. 2011 Jul;254(1):83-9.
- Dang Y, Shi X, Xu W, Zuo M. The effect of anesthesia on the immune system in colorectal cancer patients. Canadian Journal of Gastroenterology & Hepatology. 2018 Apr 1;2018:7940603; Gelman D, Gelmanas A et al. Role of multimodal analgesia in the evolving enhanced recovery after surgery pathways. Medicina. 2018 May; 54(2): 20.
- Fligor SC, Wang S, Allar BG, et al. Gastrointestinal malignancies and the COVID-19 pandemic: evidence-based triage to surgery. Journal of Gastrointestinal Surgery. 2020;1-17.
- Haldar R, Ben-Eliyahu S. Reducing the risk of post-surgical cancer recurrence: a perioperative anti-inflammatory anti-stress approach. Future Oncology. 2018;14(11):1017-1021; Mills GA, Horn JR. Beta-blockers and glucose control. Drug Intelligence and Clinical Pharmacy. 1985 Apr;19(4):246-51.
- Hiller JG, Perry NJ, Poulogiannis G, Riedel B, Sloan EK. Perioperative events influence cancer recurrence risk after surgery. Nature Reviews Clinical Oncology. 2018 Apr;15(4):205-218.
- Aoyama T, Oba K et al. Impact of postoperative complications on the colorectal cancer survival and recurrence: analyses of pooled individual patients' data from three large phase III randomized trials. Cancer Medicine. 2017;6(7):1573‐1580; Kirchhoff P, Clavien PA, Hahnloser D. Complications in colorectal surgery: risk factors and preventive strategies. Patient Safety in Surgery. 2010;4(1):5.
- Aoyama T, Oba K et al. Impact of postoperative complications on the colorectal cancer survival and recurrence: analyses of pooled individual patients' data from three large phase III randomized trials. Cancer Medicine. 2017;6(7):1573‐1580; Kirchhoff P, Clavien PA, Hahnloser D. Complications in colorectal surgery: risk factors and preventive strategies. Patient Safety in Surgery. 2010;4(1):5; Aquina CT, Mohile SG et al. The impact of age on complications, survival, and cause of death following colon cancer surgery. British Journal of Cancer. 2017;116(3):389-397; Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Care. New York: Bantam Dell. 2009.
- Anderson SW, Bazzell AF, Dains JE. An integrative review on the effect of prebiotics, probiotics, and synbiotics on infection after colorectal cancer surgery. AORN Journal. 2018;107(2):237–248.
- Kim IY, Kim BR, Kim YW. Factors affecting use and delay (≥8 weeks) of adjuvant chemotherapy after colorectal cancer surgery and the impact of chemotherapy-use and delay on oncologic outcomes. PLoS One. 2015;10(9):e0138720.
- Cata JP, Guerra CE, Chang GJ, Gottumukkala V, Joshi GP. Non-steroidal anti-inflammatory drugs in the oncological surgical population: beneficial or harmful? A systematic review of the literature. British Journal of Anaesthesia. 2017;119(4):750-764.
- Aoyama T, Oba K et al. Impact of postoperative complications on the colorectal cancer survival and recurrence: analyses of pooled individual patients' data from three large phase III randomized trials. Cancer Medicine. 2017;6(7):1573‐1580.
- Block KI. Life over Cancer: The Block Center Program for Integrative Cancer Care. New York: Bantam Dell. 2009; Aquina CT, Mohile SG et al. The impact of age on complications, survival, and cause of death following colon cancer surgery. British Journal of Cancer. 2017;116(3):389-397.
- Kirchhoff P, Clavien PA, Hahnloser D. Complications in colorectal surgery: risk factors and preventive strategies. Patient Safety in Surgery. 2010;4(1):5.
- Minnella EM, Carli F. Prehabilitation and functional recovery for colorectal cancer patients. European Journal of Surgical Oncology. 2018;44(7):919‐926.
- Kirchhoff P, Clavien PA, Hahnloser D. Complications in colorectal surgery: risk factors and preventive strategies. Patient Safety in Surgery. 2010;4(1):5.
- Minnella EM, Carli F. Prehabilitation and functional recovery for colorectal cancer patients. European Journal of Surgical Oncology. 2018;44(7):919‐926.
- Holubar SD, Brickman RK, Greaves SW, Ivatury SJ. Neoadjuvant radiotherapy: a risk factor for short-term wound complications after radical resection for rectal cancer? Journal of the American College of Surgeons. 2016;223(2):291‐298.
- Schiffmann L, Wedermann N et al. Intensified neoadjuvant radiochemotherapy for rectal cancer enhances surgical complications. BMC Surgery. 2013;13:43.
- Rutegård J, Rutegård M. Non-steroidal anti-inflammatory drugs in colorectal surgery: a risk factor for anastomotic complications? World Journal of Gastrointestinal Surgery. 2012;4(12):278-280.
- Anderson SW, Bazzell AF, Dains JE. An integrative review on the effect of prebiotics, probiotics, and synbiotics on infection after colorectal cancer surgery. AORN Journal. 2018;107(2):237–248.
- Xiao J, Caan BJ et al. Association of low muscle mass and low muscle radiodensity with morbidity and mortality for colon cancer surgery. JAMA Surgery. 2020;e202497.
- Cata JP, Guerra CE, Chang GJ, Gottumukkala V, Joshi GP. Non-steroidal anti-inflammatory drugs in the oncological surgical population: beneficial or harmful? A systematic review of the literature. British Journal of Anaesthesia. 2017;119(4):750-764; Rutegård J, Rutegård M. Non-steroidal anti-inflammatory drugs in colorectal surgery: a risk factor for anastomotic complications? World Journal of Gastrointestinal Surgery. 2012;4(12):278-280.
- Phillips B. Reducing gastrointestinal anastomotic leak rates: review of challenges and solutions. Open Access Surgery. 2016;9:5-14; Rutegård J, Rutegård M. Non-steroidal anti-inflammatory drugs in colorectal surgery: a risk factor for anastomotic complications? World Journal of Gastrointestinal Surgery. 2012;4(12):278-280; Kendrick JB, Kaye AD et al. Goal-directed fluid therapy in the perioperative setting. Journal of Anaesthesiology and Clinical Pharmacology. 2019 Apr;35(Suppl 1):S29-S34.
- Cho JS, Lee MH et al. The effects of perioperative anesthesia and analgesia on immune function in patients undergoing breast cancer resection: a prospective randomized study. International Journal of Medical Sciences. 2017 Aug 18;14(10):970-976.
- Berdah SV, Mariette C et al. A multicentre, randomised, controlled trial to assess the safety, ease of use, and reliability of hyaluronic acid/carboxymethylcellulose powder adhesion barrier versus no barrier in colorectal laparoscopic surgery. Trials. 2014;15:413.
- Kim IY, Kim BR, Kim YW. Factors affecting use and delay (≥8 weeks) of adjuvant chemotherapy after colorectal cancer surgery and the impact of chemotherapy-use and delay on oncologic outcomes. PLoS One. 2015;10(9):e0138720.
- Schiffmann L, Wedermann N et al. Intensified neoadjuvant radiochemotherapy for rectal cancer enhances surgical complications. BMC Surgery. 2013;13:43.
- Holubar SD, Brickman RK, Greaves SW, Ivatury SJ. Neoadjuvant radiotherapy: a risk factor for short-term wound complications after radical resection for rectal cancer? Journal of the American College of Surgeons. 2016;223(2):291‐298.
- O'Gorman C, Denieffe S, Gooney M. Literature review: preoperative radiotherapy and rectal cancer—impact on acute symptom presentation and quality of life. Journal of Clinical Nursing. 2014;23(3-4):333‐351.
- Schiffmann L, Wedermann N et al. Intensified neoadjuvant radiochemotherapy for rectal cancer enhances surgical complications. BMC Surgery. 2013;13:43.
- Sikorszki L, Bezsilla J, Vajda K, Temesi R. Lokálisan előrehaladott kemoirradiált rectumtumorok nyitott és laparoszkópos műtéteinek az összehasonlítása [The comparison of open and laparoscopic surgeries of the locally advanced chemo-irradiated rectum tumours]. Magyar Sebészet. 2020;73(1):29-36.
- Redmond HP, Neary PM et al. RandomiSed clinical trial assessing Use of an anti-inflammatoRy aGent in attenUating peri-operatiVe inflAmmatioN in non-meTastatic colon cancer—the S.U.R.G.U.V.A.N.T. trial. BMC Cancer. 2018;18(1):794.
- Tebala GD, Gordon-Dixon A, Imtiaz M, Shrestha A, Toeima M. Enhanced recovery after rectal surgery: what we have learned so far. Mini-invasive Surgery. 2018;2:32.
- Anderson SW, Bazzell AF, Dains JE. An integrative review on the effect of prebiotics, probiotics, and synbiotics on infection after colorectal cancer surgery. AORN Journal. 2018;107(2):237–248; Aisu N, Tanimura S et al. Impact of perioperative probiotic treatment for surgical site infections in patients with colorectal cancer. Experimental and Therapeutic Medicine. 2015;10(3):966–972.
- Tebala GD, Gordon-Dixon A, Imtiaz M, Shrestha A, Toeima M. Enhanced recovery after rectal surgery: what we have learned so far. Mini-invasive Surgery. 2018;2:32.
- Tebala GD, Gordon-Dixon A, Imtiaz M, Shrestha A, Toeima M. Enhanced recovery after rectal surgery: what we have learned so far. Mini-invasive Surgery. 2018;2:32.
- Gelman D, Gelmanas A, Urbanaitė D, et al. Role of multimodal analgesia in the evolving enhanced recovery after surgery pathways. Medicina (Kaunas). 2018;54(2):20.
- Kim SY, Kim NK et al. Effects of postoperative pain management on immune function after laparoscopic resection of colorectal cancer: a randomized study [published correction appears in Medicine (Baltimore). 2016 Jun 17;95(24):e4641]. Medicine (Baltimore). 2016;95(19):e3602.
- Kim SY, Kim NK et al. Effects of postoperative pain management on immune function after laparoscopic resection of colorectal cancer: a randomized study [published correction appears in Medicine (Baltimore). 2016 Jun 17;95(24):e4641]. Medicine (Baltimore). 2016;95(19):e3602.
- Radovanović D, Radovanović Z et al. Thoracic epidural versus intravenous patient-controlled analgesia after open colorectal cancer surgery. Acta Clinica Croatia. 2017;56(2):244‐254.
- Deng W, Long X et al. Quadratus lumborum block versus transversus abdominis plane block for postoperative pain management after laparoscopic colorectal surgery: a randomized controlled trial. Medicine (Baltimore). 2019;98(52):e18448.
- Damadi AA, Lax EA, Smithson L, Pearlman RD. Comparison of therapeutic benefit of bupivacaine hcl transversus abdominis plane (TAP) block as part of an enhanced recovery pathway versus traditional oral and intravenous pain control after minimally invasive colorectal surgery: a prospective, randomized, double-blind trial. American Surgeon. 2019;85(12):1363-1368.
- Keller DS, Tahilramani RN et al. Pilot study of a novel pain management strategy: evaluating the impact on patient outcomes. Surgical Endoscopy. 2016;30(6):2192-2198.
- Pandazi A, Kapota E et al. Preincisional versus postincisional administration of parecoxib in colorectal surgery: effect on postoperative pain control and cytokine response. A randomized clinical trial. World Journal of Surgery. 2010;34(10):2463-2469.
- Yin LH, Li WS, Zhao WX, Li WY. [Role of acupuncture anesthesia in operation of rectal cancer] [Article in Chinese]. Zhongguo Zhen Jiu. 2005;25(12):876-878.
- Huang W, Yu TY, Long WF, Xiao JB. [Application of transcutaneous electrical acupoint stimulation combined with transversus abdominis plane block to enhanced recovery after surgery in patients undergoing laparoscopic colorectal cancer resection: a randomized controlled clinical trial] [Article in Chinese]. Zhen Ci Yan Jiu. 2018;43(10):611-615.
- Jamjittrong S, Matsuda A et al. Postoperative non-steroidal anti-inflammatory drugs and anastomotic leakage after gastrointestinal anastomoses: systematic review and meta-analysis. Annals of Gastroenterological Surgery. 2019;4(1):64-75; Cata JP, Guerra CE, Chang GJ, Gottumukkala V, Joshi GP. Non-steroidal anti-inflammatory drugs in the oncological surgical population: beneficial or harmful? A systematic review of the literature. British Journal of Anaesthesia. 2017 Oct 1;119(4):750-764.
- Cata JP, Guerra CE, Chang GJ, Gottumukkala V, Joshi GP. Non-steroidal anti-inflammatory drugs in the oncological surgical population: beneficial or harmful? A systematic review of the literature. British Journal of Anaesthesia. 2017;119(4):750-764.
- Liu Y, May BH et al. Acupuncture and related therapies for treatment of postoperative ileus in colorectal cancer: a systematic review and meta-analysis of randomized controlled trials. Evidence-Based Complementary and Alternative Medicine. 2018;2018:3178472; Kim KH, Kim DH, Kim HY, Son GM. Acupuncture for recovery after surgery in patients undergoing colorectal cancer resection: a systematic review and meta-analysis. Acupuncture in Medicine. 2016;34(4):248-256.
- Mai S, Meng J, Wang W, Lang S. [Influence of electroacupuncture pretreatment on intestinal function in the patients of colorectal cancer surgery] [Article in Chinese]. Zhongguo Zhen Jiu. 2017;37(5):483-487.
- Yang Y, Zuo HQ et al. Comparison of efficacy of simo decoction and acupuncture or chewing gum alone on postoperative ileus in colorectal cancer resection: a randomized trial. Scientific Reports. 2017;7:37826.
- Ng SS, Leung WW et al. Electroacupuncture reduces duration of postoperative ileus after laparoscopic surgery for colorectal cancer. Gastroenterology. 2013;144(2):307-313.e1.
- Ng SS, Leung WW et al. Electroacupuncture reduces duration of postoperative ileus after laparoscopic surgery for colorectal cancer. Gastroenterology. 2013;144(2):307-313.e1.
- Ng SS, Leung WW et al. Electroacupuncture reduces duration of postoperative ileus after laparoscopic surgery for colorectal cancer. Gastroenterology. 2013;144(2):307-313.e1.
- Wang TY, Meng JH, Mai SC. [Electroacupuncture treatment conducted before and after surgery is better in promoting reco-very of gastrointestinal function in colorectal cancer patients undergoing radical resection] [Article in Chinese]. Zhen Ci Yan Jiu. 2018;43(12):797-800.
- Guo J, Tang W et al. [Transcutaneous electrical acupoint stimulation on inflammatory response and intestinal permeability in perioperative period of laparoscopic intestinal surgery] [Article in Chinese]. Zhongguo Zhen Jiu. 2018;38(10):1043-1046.
- Huang W, Yu TY, Long WF, Xiao JB. [Application of transcutaneous electrical acupoint stimulation combined with transversus abdominis plane block to enhanced recovery after surgery in patients undergoing laparoscopic colorectal cancer resection: a randomized controlled clinical trial] [Article in Chinese]. Zhen Ci Yan Jiu. 2018;43(10):611-615.
- Tebala GD, Gordon-Dixon A, Imtiaz M, Shrestha A, Toeima M. Enhanced recovery after rectal surgery: what we have learned so far. Mini-invasive Surgery. 2018;2:32.
- Gan T, Jackson NA et al. A retrospective review: patient-reported preoperative prescription opioid, sedative, or antidepressant use is associated with worse outcomes in colorectal surgery. Diseases of the Colon & Rectum. 2020;63(7):965-973.
- Brand JM, Kirchner H, Poppe C, Schmucker P. The effects of general anesthesia on human peripheral immune cell distribution and cytokine production. Clinical Immunology and Immunopathology. 1997;83(2):190-194.
- Hiller J, Brodner G, Gottschalk A. Understanding clinical strategies that may impact tumour growth and metastatic spread at the time of cancer surgery. Best Practice & Research Clinical Anaesthesiology. 2013;27(4):427-439; Dang Y, Shi X, Xu W, Zuo M. The effect of anesthesia on the immune system in colorectal cancer patients. Gastroenterological Cancer and Immunotherapy. 2018;2018:7940603; Khanna AK, Riveros Perez E, Laudanski K, Moraska A, Cummings III KC. Perioperative care and cancer recurrence: is there a connection? World Journal of Anesthesiology. 2014 Mar 27;3(1):31-45.
- Khanna AK, Riveros Perez E, Laudanski K, Moraska A, Cummings III KC. Perioperative care and cancer recurrence: is there a connection? World Journal of Anesthesiology. 2014 Mar 27;3(1):31-45.
- Lee SK, Dawson J et al. Management of cancer pain: 1. Wider implications of orthodox analgesics. International Journal of General Medicine. 2014;7:49-58.
- Day A, Smith R et al. Retrospective analysis of the effect of postoperative analgesia on survival in patients after laparoscopic resection of colorectal cancer. British Journal of Anaesthesia. 2012;109(2):185-190.
- Kim SY, Kim NK et al. Effects of postoperative pain management on immune function after laparoscopic resection of colorectal cancer: a randomized study [published correction appears in Medicine (Baltimore). 2016 Jun 17;95(24):e4641]. Medicine (Baltimore). 2016;95(19):e3602.
- Dang Y, Shi X et al. The Effect of Anesthesia on the Immune System in Colorectal Cancer Patients. Canadian Journal of Gastroenterology & Hepatology. 2018 Apr 1;2018:7940603.
- Kim SY, Kim NK et al. Effects of Postoperative Pain Management on Immune Function After Laparoscopic Resection of Colorectal Cancer: A Randomized Study. Medicine (Baltimore). 2016 Jun 17;95(24):e4641.
- Khanna AK, Riveros Perez E, Laudanski K, Moraska A, Cummings III KC. Perioperative care and cancer recurrence: is there a connection? World Journal of Anesthesiology. 2014 Mar 27;3(1):31-45.
- Beilin B, Shavit Y et al. Effects of anesthesia based on large versus small doses of fentanyl on natural killer cell cytotoxicity in the perioperative period. Anesthesia and Analgesia. 1996 Mar;82(3):492-7.
- Biki B, Mascha E et al. Anesthetic technique for radical prostatectomy surgery affects cancer recurrence: a retrospective analysis. Anesthesiology. 2008;109(2):180-187.
- Khanna AK, Perez ER et al. Perioperative care and cancer recurrence: Is there a connection? World Journal of Anesthesiology. 2014;3(1):31-45; Dang Y, Shi X et al. The Effect of Anesthesia on the Immune System in Colorectal Cancer Patients. Canadian Journal of Gastroenterology & Hepatology. 2018 Apr 1;2018:7940603.
- Diaz-Cambronero O, Mazzinari G et al. Perioperative Opioids and Colorectal Cancer Recurrence: A Systematic Review of the Literature. Pain Management. 2018 Sep 1;8(5):353-361.
- Schack A, Fransgaard T, Klein MF, Gögenur I. Perioperative use of nonsteroidal anti-inflammatory drugs decreases the risk of recurrence of cancer after colorectal resection: a cohort study based on prospective data. Annals of Surgical Oncology. 2019;26(12):3826-3837.
- Lönnroth C, Andersson M et al. Preoperative treatment with a non-steroidal anti-inflammatory drug (NSAID) increases tumor tissue infiltration of seemingly activated immune cells in colorectal cancer. Cancer Immunity. 2008;8:5.
- Schack A, Fransgaard T, Klein MF, Gögenur I. Perioperative use of nonsteroidal anti-inflammatory drugs decreases the risk of recurrence of cancer after colorectal resection: a cohort study based on prospective data. Annals of Surgical Oncology. 2019 Nov;26(12):3826-3837.
- Cata JP, Guerra CE, Chang GJ, Gottumukkala V, Joshi GP. Non-steroidal anti-inflammatory drugs in the oncological surgical population: beneficial or harmful? A systematic review of the literature. British Journal of Anaesthesia. 2017 Oct 1;119(4):750-764.
- Dang Y, Shi X et al. The effect of anesthesia on the immune system in colorectal cancer patients. Canadian Journal of Gastroenterology & Hepatology. 2018 Apr 1;2018:7940603.
- Panigrahy D, Gartung A et al. Preoperative stimulation of resolution and inflammation blockade eradicates micrometastases. Journal of Clinical Investigation. 2019;129(7):2964-2979.
- Cata JP, Guerra CE, Chang GJ, Gottumukkala V, Joshi GP. Non-steroidal anti-inflammatory drugs in the oncological surgical population: beneficial or harmful? A systematic review of the literature. British Journal of Anaesthesia. 2017 Oct 1;119(4):750-764.
- Jia N, Sun Z et al. Serial monitoring of circulating tumor DNA in patients with metastatic colorectal cancer to predict the therapeutic response. Frontiers in Genetics. 2019 May 21;10:470.
- Reece M, Saluja H et al. The use of circulating tumor DNA to monitor and predict response to treatment in colorectal cancer. Frontiers in Genetics. 2019;10:1118.
- Tie J, Cohen JD et al. Circulating tumor DNA analyses as markers of recurrence risk and benefit of adjuvant therapy for stage III colon cancer [published correction Error in Figure 2D appears in JAMA Oncology. 2019 Dec 1;5(12):1811]. JAMA Oncology. 2019;5(12):1710-1717.
- Baek DH, Kim GH et al. Clinical potential of circulating tumor cells in colorectal cancer: a prospective study. Clinical and Translational Gastroenterology. 2019;10(7):e00055.
- Yang C, Chen F, Wang S, Xiong B. Circulating tumor cells in gastrointestinal cancers: current status and future perspectives. Frontiers in Oncology. 2019;9:1427; Burz C, Pop VV et al. Circulating tumor cells in clinical research and monitoring patients with colorectal cancer. Oncotarget. 2018;9(36):24561-24571.
- Alschuler LN, Gazella KA. The Definitive Guide to Thriving after Cancer: A Five-Step Integrative Plan to Reduce the Risk of Recurrence and Build Lifelong Health. Berkeley, California: Ten Speed Press. 2013.
More Information
General Information
- National Cancer Institute: Contact Us for Help
Information specialists at the NCI Contact Center are available to help answer your cancer-related questions in English and Spanish whether you are a patient, family member or friend, health care provider, or researcher. We also respond to questions and requests for information about NCI and its programs and provide support in quitting smoking. - Goldenberg BA, Holliday EB, Helewa RM, Singh H. Rectal cancer in 2018: a primer for the gastroenterologist. American Journal of Gastroenterology 2018;113(12):1763–1771.
- Lee B: Colorectal Cancer: FOLFOX/FOLFIRI and Supportive natural therapies. Marsden Centre for Integrative Medicine
Prevention
- American Institute for Cancer Research:
- National Cancer Institute:
- Centers for Disease Control and Prevention: What Can I Do to Reduce My Risk of Colorectal Cancer?
- American Cancer Society: Can Colorectal Cancer Be Prevented?
- QCancer®(15yr,colorectal) risk calculator
- Anticancer Lifestyle Program: Q&A with Dr. Chloe Atreya, Gastrointestinal Medical Oncologist, UCSF: Keys Facts about Colorectal Cancer
Surgery
- Medscape:
- Medical Archives: Introduction to total mesorectal excision
Clinical Trials
- Curcumin: Study Investigating the Ability of Plant Exosomes to Deliver Curcumin to Normal and Colon Cancer Tissue (expected to end in late 2022)
Advocacy and Support Groups
- National Comprehensive Cancer Network: Advocacy and Support Groups; select colon cancer, rectal cancer or another topic of interest from the dropdown menu.
More from Our Resources Database
- Gurdev Parmar and Tina Kaczor: Textbook of Naturopathic Oncology
- Block KI, Block PB, Gyllenhaal C: Integrative Treatment for Colorectal Cancer
- Lise N. Alschuler and Karolyn A. Gazella: The Definitive Guide to Thriving after Cancer
- Makala Kozo Hattori: The Healing Grace of Cancer
- Danial E. Baker: Application of chronotherapy to the treatment of cancer: can changing the timing of drug administration influence efficacy and toxicity?
- Ralph Moss, PhD: The Ultimate Guide to Cancer: DIY Research
- US Department of Health and Human Services: Physical Activity Guidelines for Americans
- September 2018 Issue of the Journal of Alternative and Complementary Medicine
- Lise Alschuler, ND, FABNO, and Karolyn Gazella: The Definitive Guide to Cancer, 3rd Edition
- Keith I. Block, MD: Life over Cancer: The Block Center Program for Integrative Cancer Treatment
- Ralph Moss, PhD: The Moss Reports
Related Pages
Personal Stories
In the News
- Liquid biopsy may save colon cancer patients from chemo
- ASCO 2022: 100% complete response rate in MMRd locally advanced rectal cancer seen in pivotal ‘immunoablative’ neoadjuvant immunotherapy clinical trial
- USPSTF no longer recommends aspirin as prophylaxis for colorectal cancer
- Changes in the NCCN guidelines on colorectal cancer screening
- Minimally invasive emergent colorectal surgery tied to better outcomes



 Nancy Hepp, MS, BCCT Project Manager
Nancy Hepp, MS, BCCT Project Manager
